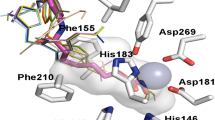
Abstract
Colorectal cancer (CRC) is a frequent malignancy with a poor prognosis and a high fatality rate, unlike other malignancies. Structural improvements have been developed to better comprehend the binding of small-molecule inhibitors, reducing the side effects of chemotherapy medications. A significant functional group extensively present in both pharmacological medicines and natural compounds is the nitrile group; consequently, novel treatment compounds are desperately needed. This study aimed to design and synthesize novel anticancer drug candidates and then examine their anticancer activity against human cancer cell lines. The anti-proliferation of human colon cancer cells (HCT116) was examined using the MMT assay and compared to the activity of doxorubicin as chemotherapy after characterizing novel derivatives of di (2-aryl hydrazonopropane) arene by elemental analyzer, FTIR, 1H, 13C NMR, and ESI-MS. Chemoinformatic tools predicted their targets, and then molecular docking was performed to predict the binding affinity of compounds to the main receptors expressed in colon cancer: the adenosine receptor (A2AR) and the low-density lipoprotein receptor-related protein (LRP6). To form the corresponding diaryl hydrazone coupling products from the di-(2-aryl hydrazonopropane) arene derivatives, equivalent amounts of enaminones were coupled with diazotized aniline derivatives. Compound 11b, containing a benzonitrile moiety, was the most potent against HCT116 cells (IC50 of 0.098 ± 0.03 μM) and had a greater binding affinity to both A2AR and LRP6 of −12.43 and −16.45 kcal/mol, respectively. Our results demonstrated that the dibenzonitrile moiety is a good part of compounds that are candidates as anticancer drugs against HCT116 cells.





Similar content being viewed by others
References
Bakhsh T, Alhazmi S, Alburae NA, Farsi A, Alzahrani F, Choudhry H, et al. Exosomal miRNAs as a promising source of biomarkers in colorectal cancer progression. Int J Mol Sci. 2022;23:4855.
Alyabsi M, Algarni M, Alshammari K. Trends in colorectal cancer incidence rates in Saudi Arabia (2001–2016) using Saudi National Registry: early-versus late-onset disease. Front Oncol. 2021;11:730689.
Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev/Prz Gastroenterol. 2019;14:89–103.
Van der Jeught K, Xu H-C, Li Y-J, Lu X-B, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834.
Geng L, Wang J. Molecular effectors of radiation resistance in colorectal cancer. Precis Radiat Oncol. 2017;1:27–33.
Xie Y-H, Chen Y-X, Fang J-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
Liang L, Tu Y, Lu J, Wang P, Guo Z, Wang Q, et al. Dkk1 exacerbates doxorubicin-induced cardiotoxicity by inhibiting the Wnt/β-catenin signaling pathway. J Cell Sci. 2019;132:jcs228478.
Wang X, Wang Y, Li X, Yu Z, Song C, Du Y. Nitrile-containing pharmaceuticals: target, mechanism of action, and their SAR studies. RSC Med Chem. 2021;12:1650–71.
Fleming FF, Yao L, Ravikumar P, Funk L, Shook BC. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J Med Chem. 2010;53:7902–17.
Wild RA, Reis SE. Estrogens, progestins, selective estrogen receptor modulators, and the arterial tree. Am J Obstet Gynecol. 2001;184:1031–9.
Boyd MJ, Crane SN, Robichaud J, Scheigetz J, Black WC, Chauret N, et al. Investigation of ketone warheads as alternatives to the nitrile for preparation of potent and selective cathepsin K inhibitors. Bioorg Med Chem Lett. 2009;19:675–9.
Sindrup S, Brøsen K, Hansen M, Aaes-Jørgensen T, Overø K, Gram L. Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit. 1993;15:11–7.
Jackson T, Woo LL, Trusselle MN, Purohit A, Reed MJ, Potter BV. Non‐steroidal aromatase inhibitors based on a biphenyl scaffold: synthesis, in vitro sar, and molecular modelling. ChemMedChem. 2008;3:603–18.
Aghabozorgi AS, Ebrahimi R, Bahiraee A, Tehrani SS, Nabizadeh F, Setayesh L, et al. The genetic factors associated with Wnt signaling pathway in colorectal cancer. Life Sci. 2020;256:118006.
Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D, et al. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure. 2011;19:1433–42.
Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5.
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8.
Bhanot P, Brink M, Samos CH, Hsieh J-C, Wang Y, Macke JP, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30.
Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, et al. Reconstitution of a Frizzled8· Wnt3a· LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9.
Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS ONE. 2010;5:e12682.
Curtin JC, Lorenzi MV. Erratum: drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2018;9:34856.
Semënov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem. 2008;283:21427–32.
Dahlmann M, Monks A, Harris ED, Kobelt D, Osterland M, Khaireddine F, et al. Combination of wnt/β-catenin targets S100A4 and DKK1 improves prognosis of human colorectal cancer. Cancers. 2021;14:37.
Al-Zaydi KM, Mekheimer RA, Mousally SM, Borik RM, Elnagdi MH. An expeditious and green synthesis of new enaminones and study their chemical reactivity toward some different amines and binucleophiles under environmentally friendly conditions. Arab J Chem. 2017;10:S2697–S704.
Zaydi KA, Al-Johani M, Alqahtani N, Mousally S, Elnagdi NH. Reactions under pressure: synthesis of functionally substituted arylhydrazonal derivatives as precursors of novel pyridazines and nicotinates. Russ J Gen Chem. 2020;90:710–9.
Mak K.-K, Pichika M.R. Artificial intelligence in drug development: present status and future prospects. Drug Discov Today. 2019;24:773–780.
Popiołek Ł, Biernasiuk A. New hydrazides and hydrazide‐hydrazones of 2, 3‐dihalogen substituted propionic acids: synthesis and in vitro antimicrobial activity evaluation. Chem Biodivers. 2017;14:e1700075.
Shakdofa MM, Shtaiwi MH, Morsy N, Abdel-rassel T. Metal complexes of hydrazones and their biological, analytical and catalytic applications: a review. Main Group Chem. 2014;13:187–218.
Kajal A, Bala S, Sharma N, Kamboj S, Saini V. Therapeutic potential of hydrazones as anti-inflammatory agents. Int J Med Chem. 2014;2014:761030.
Dembitsky VM, Ermolenko E, Savidov N, Gloriozova TA, Poroikov VV. Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: origin, structures and biological activity. Molecules. 2021;26:686.
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23.
Raisch J, Côté-Biron A, Rivard N. A role for the WNT co-receptor LRP6 in pathogenesis and therapy of epithelial cancers. Cancers. 2019;11:1162.
Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W64.
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.
Ran L, Mou X, Peng Z, Li X, Li M, Xu D, et al. ADORA2A promotes proliferation and inhibits apoptosis through PI3K/AKT pathway activation in colorectal carcinoma. 2022. https://doi.org/10.21203/rs.3.rs-2224036/v1.
Pereira JC, Caffarena ER, Dos Santos CN. Boosting docking-based virtual screening with deep learning. J Chem Inf Model. 2016;56:2495–506.
Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE. 2010;5:e12029.
Majewski M, Ruiz-Carmona S, Barril X. An investigation of structural stability in protein-ligand complexes reveals the balance between order and disorder. Commun Chem. 2019;2:110.
Sun C, Wang B, Hao S. Adenosine-A2A receptor pathway in cancer immunotherapy. Front Immunol. 2022;13:837230.
Ma X-L, Shen M-N, Hu B, Wang B-L, Yang W-J, Lv L-H, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12:1–17.
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu J, et al. STC2 promotes the epithelial-mesenchymal transition of colorectal cancer cells through AKT-ERK signaling pathways. Oncotarget. 2016;7:71400.
Li Y, Bu G. LRP5/6 in Wnt signaling and tumorigenesis. 2005;1:673–81.
Al-Shiekh MA, El-Din AMS, Hafez EA, Elnagdi MH. α-Enones in heterocyclic synthesis, Part I. Classical synthetic and environmentally friendly synthetic approaches to alkyl and acyl azoles and azines. J Chem Res. 2004;2004:174–9.
Pleier A-K, Glas H, Grosche M, Sirsch P, Thiel WR. Microwave assisted synthesis of 1-aryl-3-dimethylaminoprop-2-enones: a simple and rapid access to 3 (5)-arylpyrazoles. Synthesis. 2001;2001:0055–62.
Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–10.
Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, et al. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–5.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.
Acknowledgements
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant No. (UJ-23-DR-57). The authors, therefore, thank the University of Jeddah for its technical and financial support.
United States patent
Patent No.: US10,981,872B1, Date of Patent: Apr. 20, 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakhsh, T., Saleh, T.S., Al-Saedi, D.A. et al. Design, synthesis, and in silico studies of novel di-(2-aryl hydrozonopropanal) arene derivatives as potent anticancer for targeting A2AR and LRP6 in HCT116 cell. Med Chem Res 33, 66–76 (2024). https://doi.org/10.1007/s00044-023-03164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03164-1