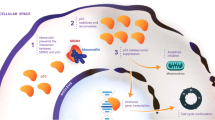
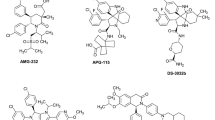
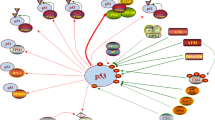
Abstract
For much of the past 20 years, MDM2 has been pursued as a cancer therapeutic target. Small molecule inhibitors that block the MDM2-p53 protein-protein interaction (MDM2 inhibitors) have been developed and a number of them have been evaluated in clinical trials for cancer treatment. Notwithstanding various setbacks, several MDM2 inhibitors have now progressed into late-stage clinical development. New strategies have also been developed to enhance the efficacy of MDM2 inhibitors and to mitigate their on-target toxicity. In this review, we summarize the progress and challenges in the development of a MDM2 targeted therapy.




Similar content being viewed by others
References
Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–32.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45.
Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55:96–107.
Juven-Gershon T, Oren M. Mdm2: the ups and downs. Mol Med. 1999;5:71–83.
Momand J, Wu HH, Dasgupta G. MDM2 - master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29.
Bond GL, Hu WW, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Tar. 2005;5:3–8.
Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83.
Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–53.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8.
Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Ding Y, Gao W, et al. Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc. 2005;127:10130–1.
Vu B, Wovkulich P, Pizzolato G, Lovey A, Ding Q, Jiang N, et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med Chem Lett. 2013;4:466–9.
Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barriere C, et al. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014;74:5855–65.
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56:5979–83.
Sun D, Li Z, Rew Y, Gribble M, Bartberger MD, Beck HP, et al. Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J Med Chem. 2014;57:1454–72.
Rew Y, Sun D. Discovery of a small molecule MDM2 inhibitor (AMG 232) for treating cancer. J Med Chem. 2014;57:6332–41.
Holzer P, Masuya K, Furet P, Kallen J, Valat-Stachyra T, Ferretti S, et al. Discovery of a Dihydroisoquinolinone Derivative (NVP-CGM097): A Highly Potent and Selective MDM2 Inhibitor Undergoing Phase 1 Clinical Trials in p53wt Tumors. J Med Chem. 2015;58:6348–58.
Holzer P. Discovery of Potent and Selective p53-MDM2 Protein-Protein Interaction Inhibitors as Anticancer Drugs. Chimia (Aarau). 2017;71:716–21.
Jeay S, Ferretti S, Holzer P, Fuchs J, Chapeau EA, Wartmann M, et al. Dose and Schedule Determine Distinct Molecular Mechanisms Underlying the Efficacy of the p53-MDM2 Inhibitor HDM201. Cancer Res. 2018;78:6257–67.
Arnhold V, Schmelz K, Proba J, Winkler A, Wunschel J, Toedling J, et al. Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma. Oncotarget. 2018;9:2304–19.
Aguilar A, Lu J, Liu L, Du D, Bernard D, McEachern D, et al. Discovery of 4-((3’R,4’S,5’R)-6”-Chloro-4’-(3-chloro-2-fluorophenyl)-1’-ethyl-2”-oxodispiro[cyclohexane-1,2’-pyrrolidine-3’,3”-indoline]-5’-carboxamido)bicyclo[2.2.2]octane -1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J Med Chem. 2017;60:2819–39.
Rudolph D, Reschke M, Blake S, Rinnenthal J, Wernitznig A, Weyer-Czernilofsky U, et al. BI 907828: A novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer Res. 2018;78:13.
Cornillie J, Wozniak A, Li H, Gebreyohannes YK, Wellens J, Hompes D, et al. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin Transl Oncol. 2020;22:546–54.
Rinnenthal J, Rudolph D, Blake S, Gollner A, Wernitznig A, Weyer-Czernilofsky U, et al. BI 907828: A highly potent MDM2 inhibitor with low human dose estimation, designed for high-dose intermittent schedules in the clinic. Cancer Res. 2018;78:13.
Kang MH, Reynolds CP, Kolb EA, Gorlick R, Carol H, Lock R, et al. Initial Testing (Stage 1) of MK-8242-A Novel MDM2 Inhibitor-by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2016;63:1744–52.
Ravandi F, Gojo I, Patnaik MM, Minden MD, Kantarjian H, Johnson-Levonas AO, et al. A phase I trial of the human double minute 2 inhibitor (MK-8242) in patients with refractory/recurrent acute myelogenous leukemia (AML). Leuk Res. 2016;48:92–100.
Konopleva M, Martinelli G, Daver N, Papayannidis C, Wei A, Higgins B, et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34:2858–74.
Andreeff M, Kelly KR, Yee K, Assouline S, Strair R, Popplewell L, et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin Cancer Res. 2016;22:868–76.
Yee K, Papayannidis C, Vey N, Dickinson MJ, Kelly KR, Assouline S, et al. Murine double minute 2 inhibition alone or with cytarabine in acute myeloid leukemia: Results from an idasanutlin phase 1/1b study small star, filled. Leuk Res. 2021;100:106489.
Italiano A, Miller WH Jr., Blay JY, Gietema JA, Bang YJ, Mileshkin LR, et al. Phase I study of daily and weekly regimens of the orally administered MDM2 antagonist idasanutlin in patients with advanced tumors. Investig New Drugs. 2021;39:1587–97.
Konopleva MY, Rollig C, Cavenagh J, Deeren D, Girshova L, Krauter J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6:4147–56.
de Weger VA, de Jonge M, Langenberg MHG, Schellens JHM, Lolkema M, Varga A, et al. A phase I study of the HDM2 antagonist SAR405838 combined with the MEK inhibitor pimasertib in patients with advanced solid tumours. Br J Cancer. 2019;120:286–93.
Jung J, Lee JS, Dickson MA, Schwartz GK, Le Cesne A, Varga A, et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat Commun. 2016;7:12609.
Hoffman-Luca CG, Ziazadeh D, McEachern D, Zhao Y, Sun W, Debussche L, et al. Elucidation of Acquired Resistance to Bcl-2 and MDM2 Inhibitors in Acute Leukemia In Vitro and In Vivo. Clin Cancer Res. 2015;21:2558–68.
Van Goethem A, Yigit N, Moreno-Smith M, Vasudevan SA, Barbieri E, Speleman F, et al. Dual targeting of MDM2 and BCL2 as a therapeutic strategy in neuroblastoma. Oncotarget. 2017;8:57047–57057.
Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, et al. Synthetic Lethality of Combined Bcl-2 Inhibition and p53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell. 2017;32:748–760.e6.
Luo Q, Pan W, Zhou S, Wang G, Yi H, Zhang L, et al. A Novel BCL-2 Inhibitor APG-2575 Exerts Synthetic Lethality With BTK or MDM2-p53 Inhibitor in Diffuse Large B-Cell Lymphoma. Oncol Res. 2020;28:331–44.
Fang DD, Tang Q, Kong Y, Wang Q, Gu J, Fang X, et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J Immunother Cancer. 2019;7:327.
Wang HQ, Liang J, Mulford I, Sharp F, Gaulis S, Chen Y, et al. Abstract 5560: PD-1/PD-L1 blockade enhances MDM2 inhibitor activity in p53 wild-type cancers. Cancer Res. 2018;78:5560.
Zhou J, Kryczek I, Li S, Li X, Aguilar A, Wei S, et al. The ubiquitin ligase MDM2 sustains STAT5 stability to control T cell-mediated antitumor immunity. Nat Immunol. 2021;22:460–70.
ASCO 2022: Ascentage Pharma Releases Updated Results Demonstrating the Therapeutic Potential of Alrizomadlin (APG-115) plus Pembrolizumab in Patients with Solid Tumors who Progressed on Immunotherapies. https://www.prnewswire.com/news-releases/asco-2022--ascentage-pharma-releases-updated-results-demonstrating-the-therapeutic-potential-of-alrizomadlin-apg-115-plus-pembrolizumab-in-patients-with-solid-tumors-who-progressed-on-immunotherapies-301562392.html.
Mullard A. p53 programmes plough on. Nat Rev Drug Discov. 2020;19:497–500.
Schoffski P, Lahmar M, Lucarelli A, Maki RG. Brightline-1: phase II/III trial of the MDM2-p53 antagonist BI 907828 versus doxorubicin in patients with advanced DDLPS. Future Oncol.2023;19:621–29.
Gounder MM, Bauer TM, Schwartz GK, Weise AM, LoRusso P, Kumar P, et al. A First-in-Human Phase I Study of Milademetan, an MDM2 Inhibitor, in Patients With Advanced Liposarcoma, Solid Tumors, or Lymphomas. J Clin Oncol. 2023;41:1714–24.
Verstovsek S, Al-Ali HK, Mascarenhas J, Perkins A, Vannucchi AM, Mohan SR, et al. BOREAS: a global, phase III study of the MDM2 inhibitor navtemadlin (KRT-232) in relapsed/refractory myelofibrosis. Future Oncol.2022;18:4059–69.
Chan KH, Zengerle M, Testa A, Ciulli A. Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds. J Med Chem. 2018;61:504–13.
Gadd MS, Testa A, Lucas X, Chan KH, Chen W, Lamont DJ, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13:514–21.
Bondeson DP, Mares A, Smith IE, Ko E, Campos S, Miah AH, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11:611–7.
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. P Natl Acad Sci USA. 2001;98:8554–9.
Schneekloth JS Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, et al. Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc. 2004;126:3748–54.
Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–14.
Crews CM, Georg G, Wang SM. Inducing Protein Degradation as a Therapeutic Strategy. J Med Chem. 2016;59:5129–30.
Bekes M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181–200.
Li Y, Yang J, Aguilar A, McEachern D, Przybranowski S, Liu L, et al. Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression. J Med Chem. 2019;62:448–66.
Wang B, Wu S, Liu J, Yang K, Xie H, Tang W. Development of selective small molecule MDM2 degraders based on nutlin. Eur J Med Chem. 2019;176:476–91.
Wang B, Liu J, Tandon I, Wu S, Teng P, Liao J, et al. Development of MDM2 degraders based on ligands derived from Ugi reactions: Lessons and discoveries. Eur J Med Chem. 2021;219:113425.
Marcellino B, Yang XB, Chen H, Chen K, Brady C, Clementelli C, et al. Development of an MDM2 Degrader for Treatment of Acute Leukemias. Blood. 2021;138:1866–+.
Chutake Y, Mayo M, Chen D, Enerson B, Cho P, Filiatrault J, et al. Abstract 3934: KT-253, a highly potent and selective heterobifunctional MDM2 degrader for the treatment of wildtype p53 tumors with superior potency and differentiated biological activity compared to small molecule inhibitors (SMI). Cancer Res. 2022;82:3934.
Mayo M, Karnik YC, McDonald A, Cho P, Filiatrault J, Chen D, et al. Development of KT-253, a Highly Potent and Selective Heterobifunctional MDM2 Degrader for the treatment of Acute Myeloid Leukemia. 64th Annual Meeting of the American Society of Hematology (ASH), December 10–13, 2022, New Orleans, LA. https://www.kymeratx.com/wp-content/uploads/2022/12/POSTER-Kymera_ASH_2022_Mayo-1.pdf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SW and AA are co-inventors of an MDM2 inhibitor APG-115 and MDM2 degraders, which have been licensed by Ascentage Pharma Group for clinical development and they receive royalty from the University of Michigan. SW is a co-founder of Ascentage, owns stock in Ascentage and is a paid consultant of Ascentage. The University of Michigan also has a research contract with Ascentage.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aguilar, A., Thomas, J.E. & Wang, S. Targeting MDM2 for the development of a new cancer therapy: progress and challenges. Med Chem Res 32, 1334–1344 (2023). https://doi.org/10.1007/s00044-023-03102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03102-1