Abstract
The phenyl-substituted thiourea derivatives (2-21) of memantine (1) were synthesized through a sonication-assisted single-step chemical reaction. Urease inhibition and cytotoxicity against 3T3 mouse fibroblast cells were also performed. The synthesized compounds were characterized using UV/VIS, FT-IR, 1H-NMR, 13C-NMR (BB, DEPT-90, DEPT-135), and mass spectrometric techniques. All of these compounds were found to be new, except 2, 14, 17, and 21. These compounds showed good activity against the urease (IC50 = 36.5 ± 0.20 to 5.6 ± 0.30 µM) while acetohydroxamic acid (AHA) was used as a standard (IC50 = 20.43 ± 0.43 µM). Compounds 6, 7, 12, and 20 were found to be the most potent urease inhibitors. Among all compounds, 5, 7, 11, 16, and 17-21 were found to be toxic against 3T3 mouse fibroblast cells, doxorubicin (IC50 = 0.1 ± 0.002 µM) was used as a standard. Urease inhibition activity of the parent drug memantine (1) was also evaluated and interestingly to the best of our knowledge we are reporting the repurposing of memantine as a urease inhibitor for the first time. (IC50 = 44.3 ± 0.52 µM). Memantine was also found to be noncytotoxic against 3T3 normal cells. The kinetics and molecular docking studies were performed for potent urease inhibitors 6, 7, 12, and 20. These compounds exhibited enhanced interaction with the active site residues of the urease in a competitive manner (Ki = 4.1-8.6 µM). Among these, compounds 6 and 12 from this library have the potential to be studied as anti-ulcer agents.
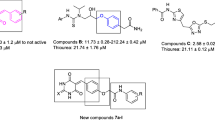
Graphical Abstract










Similar content being viewed by others
References
Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, et al. The NCGC pharmaceutical collection: A comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med. 2011;3:80ps16–80ps16.
Sardana D, Zhu C, Zhang M, Gudivada RC, Yang L, Jegga AG. Drug repositioning for orphan diseases. Brief Bioinform. 2011;12:346–56.
Ün İ, İbişoğlu H, Kılıç A, Ün ŞŞ, Yuksel F. Nucleophilic substitution reactions of adamantane derivatives with cyclophosphazenes. Inorg Chim Acta. 2012;387:226–33.
Arshad N, Saeed A, Perveen F, Ujan R, Farooqi SI, Channar PA, et al. Synthesis, X-ray, Hirshfeld surface analysis, exploration of DNA binding, urease enzyme inhibition and anticancer activities of novel adamantane-naphthyl thiourea conjugate. Bioorg Chem. 2021;109:104707.
Spilovska K, Zemek F, Korabecny J, Nepovimova E, Soukup O, Windisch M, et al. Adamantane–a lead structure for drugs in clinical practice. Curr Med Chem 2016;23:3245–66.
de Roin S, Winters S. Amantadine hydrochloride: current and new uses. Can J Neurosci Nurs. 1990;22:322–5.
Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 2004;3:109–10.
Scholtissek C, Webster RG. Long-term stability of the anti-influenza A compounds—amantadine and rimantadine. Antivir Res. 1998;38:213–5.
Stamataros G, Schneider SH. Vildagliptin in the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2011;12:1967–73.
Shakeel A, Altaf AA, Qureshi AM, Badshah A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J Drug Des Med Chem. 2016;2:10.
Sun YL, Wei Y, Shi M. Applications of chiral thiourea‐amine/phosphine organocatalysts in catalytic asymmetric reactions. ChemCatChem. 2017;9:718–27.
Burmistrov V, Morisseau C, Pitushkin D, Karlov D, Fayzullin RR, Butov GM, et al. Adamantyl thioureas as soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2018;28:2302–13.
Al-Mutairi AA, Alagappan K, Blacque O, Al-Alshaikh MA, El-Emam AA, Percino MJ, et al. Crystallographic and theoretical exploration of weak hydrogen bonds in Arylmethyl N′-(adamantan-1-yl) piperidine-1-carbothioimidates and molecular docking analysis. ACS omega. 2021;6:27026–37.
Liu J, Obando D, Liao V, Lifa T, Codd R. The many faces of the adamantyl group in drug design. Eur J Med Chem. 2011;46:1949–63.
Mazzei L, Cianci M, Vara AG, Ciurli S. The structure of urease inactivated by Ag (I): A new paradigm for enzyme inhibition by heavy metals. Dalton Trans. 2018;47:8240–7.
Vasiljev A, Simha P, Demisse N, Karlsson C, Randall DG, Vinnerås B. Drying fresh human urine in magnesium-doped alkaline substrates: Capture of free ammonia, inhibition of enzymatic urea hydrolysis & minimisation of chemical urea hydrolysis. J Chem Eng. 2022;428:131026.
Hassan ST, Šudomová M. The development of urease inhibitors: What opportunities exist for better treatment of Helicobacter pylori infection in children? Children 2017;4:2.
Rizvi F, Khan M, Jabeen A, Siddiqui H, Choudhary MI. Studies on isoniazid derivatives through a medicinal chemistry approach for the identification of new inhibitors of urease and inflammatory markers. Sci Rep. 2019;9:1–14.
Bailie N, Osborne C, Leininger J, Fletcher T, Johnston S, Ogburn P, et al. Teratogenic effect of acetohydroxamic acid in clinically normal beagles. Am J Vet Res. 1986;47:2604–11.
Griffith DP, Gleeson MJ, Lee H, Longuet R, Deman E, Earle N. Randomized, double-blind trial of Lithostat™(Acetohydroxamic Acid) in the palliative treatment of infection-induced urinary calculi. Eur Urol. 1991;20:243–7.
Milo S, Heylen RA, Glancy J, Williams GT, Patenall BL, Hathaway HJ, et al. A small-molecular inhibitor against Proteus mirabilis urease to treat catheter-associated urinary tract infections. Sci Rep. 2021;11:1–15.
Raza M, Siddiqui H, Khan M, Ullah S, Rizvi F, Ahmad R, et al. Ultrasonic-assisted synthesis of amantadine derivatives-in vitro urease and α-glucosidase inhibitory activities, mechanistic, and computational studies. J Mol Struct. 2022;1266:133544.
Liu Y, Myers EJ, Rydahl SA, Wang X. Ultrasonic-assisted synthesis, characterization, and application of a metal–organic framework: A green general chemistry laboratory project. J Chem Educ. 2019;96:2286–91.
Anastas PT, Warner JC. Green Chemistry. Front. 1998;640:1998.
Naseem S, Ashraf M, Khan S, Rafiq M, Kashif M, Rahman J, et al. Exploring biologically active hybrid pharmacophore N-substituted hydrazine-carbothioamides for urease inhibition: In vitro and in silico approach. Int J Biol Macromol. 2021;182:534–44.
Ahmed A, Saeed A, Ali OM, El-Bahy ZM, Channar PA, Khurshid A, et al. Exploring amantadine derivatives as urease inhibitors: Molecular docking and Structure–Activity Relationship (SAR) studies. Molecules 2021;26:7150.
Rizvi F, Khan M, Jabeen A, Siddiqui H, Choudhary MI. Studies on isoniazid derivatives through a medicinal chemistry approach for the identification of new inhibitors of urease and inflammatory markers. Sci Rep. 2019;9:6738.
Todd MJ, Hausinger RP. Fluoride inhibition of Klebsiella aerogenes urease: Mechanistic implications of a pseudo-uncompetitive, slow-binding inhibitor. Biochemistry 2000;39:5389–96.
Siddiqui H, Bashir MA, Javaid K, Nizamani A, Bano H, Yousuf S, et al. Ultrasonic synthesis of tyramine derivatives as novel inhibitors of α-glucosidase in vitro. J Enzym Inhib Med Chem. 2016;31:1392–403.
Pitushkin D, Burmistrov V, Butov G. Synthesis and Properties of N-(R-Adamantan-1-ylalkyl)-N′-[3 (4)-fluorophenyl] thioureas—Target-Oriented Human Soluble Epoxide Hydrolase (hsEH) Inhibitors. Russ J Org Chem. 2018;54:1469–74.
Burmistrov V, Morisseau C, D’yachenko V, Rybakov VB, Butov GM, Hammock BD. Fluoroaromatic fragments on 1, 3-disubstituted ureas enhance soluble epoxide hydrolase inhibition. J Fluor Chem. 2019;220:48–53.
Weatherburn M. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–4.
Price P, McMillan TJ. Use of the tetrazolium assay in measuring the response of human tumor cells to ionizing radiation. Cancer Res. 1990;50:1392–6.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Leatherbarrow RJ. GraFit Version 7. Horley UK: Erithacus Software Ltd; 2009.
Acknowledgements
We are thankful to Miss Kiran Fida (Cell Culture Facility Manager at the Dr. Panjwani Center for Molecular Medicine and Drug Research, ICCBS, University of Karachi) for conducting the cytotoxicity assay.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shehzad, W., Khan, M., Siddiqui, H. et al. Memantine derived compounds as potent in vitro inhibitors of urease: Repurposing of memantine, sonication assisted derivatization and in vitro enzyme inhibition, kinetics and molecular docking studies. Med Chem Res 32, 525–541 (2023). https://doi.org/10.1007/s00044-023-03020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03020-2