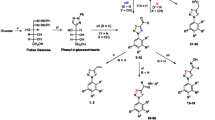
Abstract
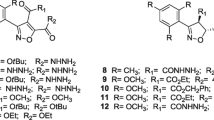
Trypanosomiasis, leishmaniasis, and malaria are tropical diseases that affect poor populations and the commonly-used drugs are toxic and can be subject to drug resistance. There is, therefore, an urgent need to find effective therapeutic agents accessible to these populations that bear the brunt of morbidity and mortality. In vitro activities of seven 1,2,3-triazenes (1a, 1b, 2a, 2b, 3a, 3b, and 4) and five 1,2,3-triazoles (5a, 5b, 6a, 6b, and 7) were evaluated against Trypanosoma brucei gambiense, Leishmania donovani, and Plasmodium falciparum. The 1,2,3-triazenes showed better activities than 1,2,3-triazoles against Leishmania donovani: 2b was the most effective against axenic amastigote forms of Leishmania donovani LV9 (IC50 = 0.89 ± 0.41 μM) and the intramacrophage-amastigote forms (IC50 = 0.99 ± 0.36 μM) with a selectivity index >100. The best trypanocidal activities against Trypanosoma brucei gambiense was observed with the 1,2,3-triazene 3a (IC50 = 3.45 ± 0.09 μM) and the triazole 7, an analog of the cholesterol side chain (IC50 = 3.99 ± 0.23 μM). The most interesting result against Plasmodium falciparum 3D7 strain was obtained with 5b (IC50 = 1.29 ± 0.15 μM) with a selectivity index >77. This study revealed that 1,2,3-triazenes and 1,2,3-triazoles show antiparasitic potential that could lead to a new class of drugs.
Graphical abstract


Similar content being viewed by others
References
Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Neglect Trop Dis. 2007;1:e114.
Tabel H, Wei G, Shi M. T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev. 2008;225:128–39.
Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, et al. The trypanosomiases. Lancet. 2003;362:1469–80.
Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasites Vectors. 2010;3:1–9.
Papageorgiou L, Megalooikonomou V, Vlachakis D. Genetic and structural study of DNA-directed RNA polymerase II of Trypanosoma brucei, towardsthe designing of novel antiparasitic agents. PeerJ 2017;5:e3061.
Komolafe MA, Sanusi AA, Idowu AO, Balogun SA, Olorunmonteni OE, Adebowale AA, et al. Sleep medicine in Africa: past, present, and future. J Clin Sleep Med. 2021;17:1317–21.
Delespaux V, de Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resistance Updates. 2007;10:30–50.
P De Koning H. The drugs of sleeping sickness: their mechanisms of action and resistance, and a brief history. Trop Med Infect Dis. 2020;5:14.
Peregrine AS. Chemotherapy and delivery systems: haemoparasites. Vet Parasitol. 1994;54:223–48.
Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82.
Sundar S, Chakravarty J. Leishmaniasis: challenges in the control and eradication. Challenges Infect Dis. Springer; 2013. p. 247–64
Pearson RD, de Queiroz Sousa A. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22:1–13.
Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55:543–50.
Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine 18E Vol 2 EB: McGraw Hill Professional; 2012.
Levaique H, Pamlard O, Apel C, Bignon J, Arriola M, Kuhner R, et al. Alkyl-resorcinol derivatives as inhibitors of GDP-mannose pyrophosphorylase with antileishmanial activities. Molecules. 2021;26:1551.
Rosenthal E, Delaunay P, Jeandel P-Y, Haas H, Pomares-Estran C, Marty P. Le traitement de la leishmaniose viscérale en Europe en 2009. Place de l’amphotéricine B liposomale. Méd Maladies Infect. 2009;39:741–4.
Guerrant RL, Walker DH, Weller PF. Tropical infectious diseases: principles, pathogens and practice e-Book: Elsevier Health Sciences; 2011.
Den Boer M, Argaw D, Jannin J, Alvar J. Leishmaniasis impact and treatment access. Clin Microbiol Infect. 2011;17:1471–7.
White Jr AC, Atmar RL, Greenberg SB. Tropical infectious diseases: principles, pathogens, and practice. American College of Physicians; 2000.
Organization WH. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010: World Health Organization; 2010.
Croft SL, Coombs GH. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–8.
Steketee RW, Choi M, Linn A, Florey L, Murphy M, Panjabi R. World Malaria Day 2021: Commemorating 15 years of contribution by the United States President’s Malaria initiative. Am J Trop Med Hyg. 2021;104(6):1955–59 https://doi.org/10.4269/ajtmh.21-0432.
Alonso P, Noor AM. The global fight against malaria is at crossroads. Lancet. 2017;390:2532–4.
Bagavan A, Rahuman AA, Kaushik NK, Sahal D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res. 2011;108:15–22.
Ombaka A. Antibacterial and antifungal activities of novel hydroxytriazenes. J Environ Chem Ecotoxicol. 2012;4:133–6.
Kimball DB, Haley MM. Triazenes: a versatile tool in organic synthesis. Angew Chem Int Ed. 2002;41:3338–51.
Hörner M, Giglio VF, Santos AJRWAD, Westphalen AB, Iglesias BA, Martins PR, et al. Triazenes and antibacterial activity. Rev Brasileira Ciências Farmêuticas. 2008;44:441–9.
Arrhenius K, Brown AS, van der Veen AM. Suitability of different containers for the sampling and storage of biogas and biomethane for the determination of the trace-level impurities–a review. Anal Chim Acta. 2016;902:22–32.
Seck I, Ndoye SF, Ba LA, Fall A, Diop A, Ciss I, et al. Access to a library of 1, 3-disubstituted-1, 2, 3-triazenes and evaluation of their antimicrobial properties. Curr Top Med Chem. 2020;20:713–9.
Insa S, Alioune F, Aicha BL, Fama NS, Seydou K, Abdoulaye D, et al. synthesis, characterization and antimicrobial activities of 1, 4-disubstituted 1, 2, 3-triazole compounds. Curr Top Med Chem. 2020;20:2289–99.
Sanada M, Takagi Y, Ito R, Sekiguchi M. Killing and mutagenic actions of dacarbazine, a chemotherapeutic alkylating agent, on human and mouse cells: effects of Mgmt and Mlh1 mutations. DNA Repair. 2004;3:413–20.
Barceló F, Ortiz-Lombardı́a M, Portugal J. Heterogeneous DNA binding modes of berenil. Biochim Biophys Acta (BBA)-Gene Struct Expr. 2001;1519:175–84.
Katsoulas A, Rachid Z, Brahimi F, McNamee J, Jean-Claude BJ. Engineering 3-alkyltriazenes to block bcr-abl kinase: a novel strategy for the therapy of advanced bcr-abl expressing leukemias. Leuk Res. 2005;29:693–700.
Iglesias BA, Hörner M, Toma HE, Araki K. 5-(1-(4-phenyl)-3-(4-nitrophenyl) triazene)-10, 15, 20-triphenylporphyrin: a new triazene-porphyrin dye and its spectroelectrochemical properties. J Porphyr Phthalocyanines. 2012;16:200–9.
Boechat N, Ferreira VF, Ferreira SB, Ferreira MDLG, da Silva FDC, Bastos MM, et al. Novel 1, 2, 3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain. J Med Chem. 2011;54:5988–99.
Costa MS, Boechat N, Rangel EA, Da Silva FdC, De Souza AM, Rodrigues CR. et al. Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1, 2, 3-triazole derivatives. Bioorg Med Chem. 2006;14:8644–53.
Nahrwold M, Bogner T, Eissler S, Verma S, Sewald N. “Clicktophycin-52”: a bioactive cryptophycin-52 triazole analogue. Org Lett. 2010;12:1064–7.
Abdel-Wahab BF, Mohamed HA, Awad GE. Synthesis and biological activity of some new 1, 2, 3-triazole hydrazone derivatives. Eur Chem Bull. 2015;4:106–9.
Seck I, Fall A, Lago C, Sene M, Gaye M, Seck M, et al. Synthesis of a cholesterol side-chain triazole analogue via ‘Click’ chemistry. Synthesis 2015;47:2826–30.
Alkhaldi AA, Abdelgawad MA, Youssif BG, El-Gendy AO, De Koning HP. Synthesis, antimicrobial activities and GAPDH docking of novel 1, 2, 3-triazole derivatives. Tropical J Pharm Res. 2019;18:1101–8.
Pocnet C, Dupuis M, Congard A, Jopp D. Personality and its links to quality of life: Mediating effects of emotion regulation and self-efficacy beliefs. Motiv Emot. 2017;41:196–208.
Devender N, Gunjan S, Tripathi R, Tripathi RP. Synthesis and antiplasmodial activity of novel indoleamide derivatives bearing sulfonamide and triazole pharmacophores. Eur J Med Chem. 2017;131:171–84.
Joshi AA, Narkhede SS, Viswanathan C. Design, synthesis and evaluation of 5-substituted amino-2, 4-diamino-8-chloropyrimido-[4, 5-b] quinolines as novel antimalarials. Bioorg Med Chem Lett. 2005;15:73–6.
Jagu E, Pomel S, Diez-Martinez A, Rascol E, Pethe S, Loiseau PM, et al. Synthesis and antikinetoplastid evaluation of bis (benzyl) spermidine derivatives. Eur J Med Chem. 2018;150:655–66.
El Ghozlani M, Bouissane L, Berkani M, Mojahidi S, Allam A, Menendez C, et al. Synthesis and biological evaluation against Leishmania donovani of novel hybrid molecules containing indazole-based 2-pyrone scaffolds. MedChemComm. 2019;10:120–7.
Balaraman K, Vieira NC, Moussa F, Vacus J, Cojean S, Pomel S, et al. In vitro and in vivo antileishmanial properties of a 2-n-propylquinoline hydroxypropyl β-cyclodextrin formulation and pharmacokinetics via intravenous route. Biomed Pharmacother. 2015;76:127–33.
Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976;193:673–5.
Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20.
Ortiz S, Vásquez-Ocmín PG, Cojean S, Bouzidi C, Michel S, Figadere B, et al. Correlation study on methoxylation pattern of flavonoids and their heme-targeted antiplasmodial activity. Bioorg Chem. 2020;104:104243.
Acknowledgements
The authors would like to thank the FIRST (Fonds impulsion pour la Recherche Scientifique et Technique) of the Senegalese Government and the Xunta de Galicia, Spain: (ED431C2017/70) and CITACA Strategic Partnership (ED431E 2018/07) for providing financial assistance for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seck, I., Ciss, I., Diédhiou, A. et al. 1,2,3-triazenes and 1,2,3-triazoles as antileishmanial, antitrypanosomal, and antiplasmodial agents. Med Chem Res 32, 158–164 (2023). https://doi.org/10.1007/s00044-022-02994-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02994-9