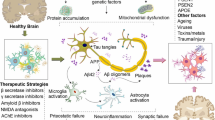
Abstract
Neurotoxicity occurs in Alzheimer’s disease due to the formation of Amyloid-β peptide aggregates and damage caused by oxidative stress. Upon aggregation of amyloid-β peptides, oxidative stress is generated; however, oxidative stress can also promote excess amyloid-β peptide production and aggregate formation. Currently available therapeutic options are little effective against Alzheimer’s disease, and they cannot stop the progression of the disease. As a new therapeutic alternative rather than inhibiting amyloid-β peptide production, inhibiting amyloid-β peptide aggregation along with oxidative stress management may be more effective considering that these processes are not typically associated with normal physiology. In addition to antiamyloidogenic properties, flavonoids exhibit antioxidant properties as well. The structural features of flavonoids that are needed for these two activities are similar. Even oxidized flavonoids are more likely to inhibit the aggregation of Amyloid-β peptides. Thus, the discovery of flavonoids with superior antioxidant activity could lead to the identification of better aggregation inhibitors. Despite flavonoids having the potential to be used as drugs, there are no medications that can be used to treat Alzheimer’s disease. This review describes how the structural features of different flavonoids affect their antiamyloidogenic and antioxidant activities, which may help develop future therapeutics for Alzheimer’s disease.

Graphical abstract




Similar content being viewed by others
References
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–1855. https://doi.org/10.1126/SCIENCE.1566067.
Müller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18:281–98. https://doi.org/10.1038/nrn.2017.29.
Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–8. https://doi.org/10.1016/0092-8674(93)90635-4.
Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between Aβ(1-42) and Aβ(1-40) in alzheimer’s β-amyloid fibril formation in vitro. Biochemistry. 1999;38:15514–21. https://doi.org/10.1021/bi991161m.
Morita M, Hamada T, Tendo Y, Hata T, Vestergaard MC, Takagi M. Selective localization of Alzheimer’s amyloid beta in membrane lateral compartments. Soft Mat. 2012;8:2816–19. https://doi.org/10.1039/c2sm07185a. 2816–2819.
Snow AD, Wight TN. Proteoglycans in the pathogenesis of Alzheimer’s disease and other amyloidoses. Neurobiol Aging. 1989;10:481–97. https://doi.org/10.1016/0197-4580(89)90108-5.
Terzi E, Hölzemann G, Seelig J. Self-association of β-amyloid peptide (1-40) in solution and binding to lipid membranes. J Mol Biol. 1995;252:633–42. https://doi.org/10.1006/jmbi.1995.0525.
Harper JD, Lansbury PT. Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. https://doi.org/10.1146/annurev.biochem.66.1.385.
Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. https://doi.org/10.1021/bi00069a001.
Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiol Aging. 2012;33:1484.e15–24. https://doi.org/10.1016/j.neurobiolaging.2011.12.020.
Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, et al. β-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–7. https://doi.org/10.1523/JNEUROSCI.1736-09.2009.
Murphy MP, LeVine H. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. https://doi.org/10.3233/JAD-2010-1221.
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. https://doi.org/10.1113/jphysiol.2005.103754.
Dahlgren KN, Manelli AM, Blaine Stine W, Baker LK, Krafft GA, Ladu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–53. https://doi.org/10.1074/jbc.M201750200.
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. https://doi.org/10.1038/nrm2101.
Tamagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M. The various aggregation states of β-amyloid 1-42 mediate different effects on oxidative stress, neurodegeneration, and BACE-1 expression. Free Radic Biol Med. 2006;41:202–12. https://doi.org/10.1016/j.freeradbiomed.2006.01.021.
Li Y, Zhou S, Li J, Sun Y, Hasimu H, Liu R, et al. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1-40-induced toxicity. Acta Pharm Sin B. 2015;5:47–54. https://doi.org/10.1016/j.apsb.2014.12.003.
Saido T, Leissring MA. Proteolytic degradation of amyloid β-protein. Cold Spring Harb Perspect Med. 2012;2:a006379–79. https://doi.org/10.1101/cshperspect.a006379.
Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. J Alzheimers Dis. 2013;33:S87–100. https://doi.org/10.3233/JAD-2012-129037.
Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid β-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4833–4841. https://doi.org/10.1523/jneurosci.22-12-04833.2002.
Butterfield DA, Boyd-Kimball D. Redox proteomics and amyloid β-peptide: insights into Alzheimer disease. J Neurochem. 2019;151:459–487. https://doi.org/10.1111/jnc.14589.
Baruch-Suchodolsky R, Fischer B. Aβ40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48:4354–70. https://pubs.acs.org/doi/10.1021/bi802361k.
Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–962. https://doi.org/10.1016/S0047-6374(01)00249-4.
Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol Med Trends Mol Med. 2001;7:548–54. https://doi.org/10.1016/S1471-4914(01)02173-6.
Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. https://doi.org/10.1016/S0197-4580(01)00340-2.
Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–20. https://doi.org/10.1016/S0197-4580(02)00225-7.
Murray IVJ, Sindoni ME, Axelsen PH. Promotion of oxidative lipid membrane damage by amyloid β proteins. Biochemistry. 2005;44:12606–13. https://doi.org/10.1021/bi050926p.
Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharm. 1996;127:31–64. https://doi.org/10.1007/bfb0048264.
Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–72. https://doi.org/10.1021/cr200084z.
Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–27988. https://doi.org/10.1006/nbdi.2002.0515.
Wang R, Wang S, Malter JS, Wang DS. Effects of HNE-modification induced by Aβ on neprilysin expression and activity in SH-SY5Y cells. J Neurochem. 2009;108:1072–82. http://doi.wiley.com/10.1111/j.1471-4159.2008.05855.x.
Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–10. https://doi.org/10.1021/bi061853s.
Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 2011;71:833–44. https://doi.org/10.1016/j.neuron.2011.07.001.
Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2018;6:CD001190. https://doi.org/10.1002/14651858.CD001190.pub3.
Birks JS. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;25:CD005593. https://doi.org/10.1002/14651858.CD005593.
Khoury R, Rajamanickam J, Grossberg GT. An update on the safety of current therapies for Alzheimer’s disease: focus on rivastigmine. Ther Adv Drug Saf. 2018;9:171–8. https://doi.org/10.1177/2042098617750555.
Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–17. https://doi.org/10.1126/science.7046051.
Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol. 2006;9:101–24. https://doi.org/10.1017/S1461145705005833.
Pedersen WA, Kloczewiak MA, Blusztajn JK. Amyloid β-protein reduces acetylcholine synthesis in a cell line derived from cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A. 1996;93:8068–71. https://doi.org/10.1073/pnas.93.15.8068.
Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–93. https://doi.org/10.1016/0006-8993(95)01032-7.
McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, et al. Memantine for dementia. Cochrane Database Syst Rev. 2019;3:CD003154. https://doi.org/10.1002/14651858.CD003154.pub6.
Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–92. https://doi.org/10.1038/nature04089.
Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N‐methyl‐D‐aspartate receptors on cultured mouse central neurones. J Physiol. 1988;396:515–33. https://doi.org/10.1113/jphysiol.1988.sp016975.
Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MV, et al. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans. 2009;37:1369–74. https://doi.org/10.1042/BST0371369.
Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, et al. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–72. https://doi.org/10.1016/j.ceca.2009.12.010.
Tamburri A, Dudilot A, Licea S, Bourgeois C, Boehm J. NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS One. 2013;8:e65350. https://doi.org/10.1371/journal.pone.0065350.
Birnbaum JH, Bali J, Rajendran L, Nitsch RM, Tackenberg C. Calcium flux-independent NMDA receptor activity is required for Aβ oligomer-induced synaptic loss. Cell Death Dis. 2015;6:e1791–91. https://doi.org/10.1038/cddis.2015.160.
Ali TB, Schleret TR, Reilly BM, Chen WY, Abagyan R. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PLoS One. 2015;10:e0144337. https://doi.org/10.1371/journal.pone.0144337.
Buckley JS, Salpeter SR. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32:453–67. https://doi.org/10.1007/s40266-015-0266-9.
Blanco-Silvente L, Castells X, Garre-Olmo J, Vilalta-Franch J, Saez M, Barceló MA, et al. Study of the strength of the evidence and the redundancy of the research on pharmacological treatment for Alzheimer’s disease: a cumulative meta-analysis and trial sequential analysis. Eur J Clin Pharmacol. 2019;75:1659–67. https://doi.org/10.1007/s00228-019-02742-w.
Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev. 2020;40:339–84. https://doi.org/10.1002/med.21622.
Maia MA, Sousa E. BACE-1 and γ-secretase as therapeutic targets for alzheimer’s disease. Pharmaceuticals. 2019;12:41. https://doi.org/10.3390/ph12010041.
Uddin MS, Kabir MT, Jeandet P, Mathew B, Ashraf GM, Perveen A, et al. Novel anti-Alzheimer’s therapeutic molecules targeting amyloid precursor protein processing. Oxid Med Cell Longev. 2020;2020:7039138. https://doi.org/10.1155/2020/7039138.
Zhou L, Barão S, Laga M, Bockstael K, Borgers M, Gijsen H, et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem. 2012;287:25927–40. https://doi.org/10.1074/jbc.M112.377465.
Zhu K, Peters F, Filser S, Herms J. Consequences of pharmacological BACE inhibition on synaptic structure and function. Biol Psychiatry. 2018;84:478–87. https://doi.org/10.1016/j.biopsych.2018.04.022.
Svedružić ŽM, Popović K, Šendula-Jengić V. Modulators of γ-secretase activity can facilitate the toxic side-effects and pathogenesis of Alzheimer’s disease. PLoS One. 2013;8:e50759. https://doi.org/10.1371/journal.pone.0050759.
Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–606. https://doi.org/10.1523/JNEUROSCI.2990-05.2005.
Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N. Engl J Med. 2006;355:2652–63. https://doi.org/10.1056/NEJMOA054625.
Nie Q, Du XG, Geng MY. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer’s disease. Acta Pharmacol Sin. 2011;32:545–51. https://doi.org/10.1038/aps.2011.14.
Jokar S, Khazaei S, Behnammanesh H, Shamloo A, Erfani M, Beiki D, et al. Recent advances in the design and applications of amyloid-β peptide aggregation inhibitors for Alzheimer’s disease therapy. Biophy Rev. 2019;11:901–25. https://doi.org/10.1007/s12551-019-00606-2.
Miners JS, Barua N, Kehoe PG, Gill S, Love S. Aβ-degrading enzymes: potential for treatment of alzheimer disease. J Neuropathol Exp Neurol. 2011;70:944–59. https://doi.org/10.1097/NEN.0b013e3182345e46.
Tiwari SC, Soni RM. Alzheimer’s disease pathology and oxidative stress: possible therapeutic. J Alzheimers Dis Park. 2014;4:1–10. https://doi.org/10.4172/2161-0460.1000162.
Teixeira JP, de Castro AA, Soares FV, da Cunha EFF, Ramalho TC. Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules. 2019;24:1–17. https://doi.org/10.3390/molecules24234410.
Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. https://doi.org/10.1016/S0300-483X(00)00306-1.
Kohen R. Skin antioxidants: Their role in aging and in oxidative stress - new approaches for their evaluation. Biomed Pharmacother. 1999;53:181–92. https://doi.org/10.1016/S0753-3322(99)80087-0.
Kohen R, Vellaichamy E, Hrbac J, Gati I, Tirosh O. Quantification of the overall reactive oxygen species scavenging capacity of biological fluids and tissues. Free Radic Biol Med. 2000;28:871–9. https://doi.org/10.1016/S0891-5849(00)00191-X.
Singh SK, Srikrishna S, Castellani RJ, Perry G. Antioxidants in the prevention and treatment of Alzheimer’s disease. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer International Publishing, 2017;523–553. https://doi.org/10.1007/978-3-319-67625-8_20.
Song K, Li Y, Zhang H, An N, Wei Y, Wang L, et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxid Med Cell Longev. 2020;2020:1–27. https://doi.org/10.1155/2020/4356386.
Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87:172–81. https://doi.org/10.1046/j.1471-4159.2003.01976.x.
Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J Agric Food Chem. 2005;53:8537–41. https://doi.org/10.1021/jf051985c.
Akaishi T, Morimoto T, Shibao M, Watanabe S, Sakai-Kato K, Utsunomiya-Tate N, et al. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid β protein. Neurosci Lett. 2008;444:280–5. https://doi.org/10.1016/j.neulet.2008.08.052.
Lee HJ, Kerr RA, Korshavn KJ, Lee J, Kang J, Ramamoorthy A, et al. Effects of hydroxyl group variations on a flavonoid backbone toward modulation of metal-free and metal-induced amyloid-β aggregation. Inorg Chem Front. 2016;3:381–92. https://doi.org/10.1039/c5qi00219b.
Sato M, Murakami K, Uno M, Nakagawa Y, Katayama S, Akagi K, et al. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the lys residues. J Biol Chem. 2013;288:23212–24. https://doi.org/10.1074/jbc.M113.464222.
Toda T, Shimizu T, Sunagawa T, Kanda T, Tagashira M, Shirasawa T. Apple procyanidins suppress amyloid β-protein aggregation. Biochem Res Int. 2011;2011:784698. https://doi.org/10.1155/2011/784698.
Thapa A, Woo ER, Chi EY, Sharoar MG, Jin HG, Shin SY, et al. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-β toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry. 2011;50:2445–55. https://doi.org/10.1021/bi101731d.
Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against β-amyloid-induced toxicity. Eur J Neurosci. 2006;23:55–64. https://doi.org/10.1111/j.1460-9568.2005.04532.x.
Liu Y, Liu Y, Wang S, Dong S, Chang P, Jiang Z. Structural characteristics of (−)-epigallocatechin-3-gallate inhibiting amyloid Aβ42 aggregation and remodeling amyloid fibers. RSC Adv. 2015;5:62402–13. https://doi.org/10.1039/c5ra09608a.
Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. https://doi.org/10.1021/np9904509.
Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta - Gen Subj. 2005;1721:174–84. https://doi.org/10.1016/j.bbagen.2004.11.001.
Dutta MS, Mahapatra P, Ghosh A, Basu S. Estimation of the reducing power and electrochemical behavior of few flavonoids and polyhydroxybenzophenones substantiated by bond dissociation energy: a comparative analysis. Mol. Divers. 2021; https://doi.org/10.1007/s11030-021-10232-4.
Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–6. https://doi.org/10.1021/np970237h.
Treml J, Šmejkal K. Flavonoids as potent scavengers of hydroxyl radicals. Compr Rev Food Sci Food Saf. 2016;15:720–38. https://doi.org/10.1111/1541-4337.12204.
Haenen GRMM, Bast A. Nitric oxide radical scavenging of flavonoids. Methods Enzymol. 1999;301:490–503. https://doi.org/10.1016/S0076-6879(99)01112-X.
Kobayashi S, Kanai S. Superoxide scavenging effects of some novel bis-ligands and their solvated metal complexes prepared by the reaction of ligands with aluminum, copper and lanthanum ions. Molecules. 2013;18:6128–41. https://doi.org/10.3390/molecules18066128.
Heijnen CGM, Haenen GRMM, Oostveen RM, Stalpers EM, Bast A. Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radic Res. 2002;36:575–81. https://doi.org/10.1080/10715760290025951.
Wang N, Li D, Lu NH, Yi L, Huang XW, Gao ZH. Peroxynitrite and hemoglobin-mediated nitrative/oxidative modification of human plasma protein: effects of some flavonoids. J Asian Nat Prod Res. 2010;12:257–64. https://doi.org/10.1080/10286021003620226.
Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997;119:99–107. https://doi.org/10.1016/S0304-3835(97)00261-9.
He J, Xu L, Yang L, Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med Sci Monit. 2018;24:8198–206. https://doi.org/10.12659/MSM.911175.
Uriarte-Pueyo I, Calvo MI. (2011) Flavonoids as acetylcholinesterase inhibitors. Curr Med Chem. 2011;18:5289–302. https://doi.org/10.2174/092986711798184325.
Balkis A, Tran K, Lee YZ, Ng K. Screening flavonoids for inhibition of acetylcholinesterase identified Baicalein as the most potent inhibitor. J Agric Sci. 2015;7:26–35. https://doi.org/10.5539/JAS.V7N9P26.
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158:47–52. https://doi.org/10.1016/S0022-510X(98)00092-6.
James SA, Churches QI, De Jonge MD, Birchall IE, Streltsov V, McColl G, et al. Iron, Copper, and Zinc concentration in Aβ plaques in the APP/PS1 mouse model of Alzheimer’s disease correlates with metal levels in the surrounding neuropil. Acs Chem Neurosci. 2017;8:629–37. https://doi.org/10.1021/ACSCHEMNEURO.6B00362.
Liu Y, Nguyen M, Robert A, Meunier B. Metal ions in Alzheimer’s disease: a key role or not? Acc Chem Res. 2019;52:2026–35. https://doi.org/10.1021/ACS.ACCOUNTS.9B00248.
Mira L, Fernandez MT, Santos M, Rocha R, Florêncio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–1208. https://doi.org/10.1080/1071576021000016463.
Detoma AS, Choi JS, Braymer JJ, Lim MH. Myricetin: a naturally occurring regulator of metal-induced amyloid-β aggregation and neurotoxicity. ChemBioChem. 2011;12:1198–201. https://doi.org/10.1002/CBIC.201000790.
Hyung SJ, Detoma AS, Brender JR, Lee S, Vivekanandan S, Kochi A, et al. Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proc Natl Acad Sci U S A. 2013;110:3743–8. https://doi.org/10.1073/PNAS.1220326110/-/DCSUPPLEMENTAL/PNAS.201220326SI.PDF.
Calcul L, Zhang B, Jinwal UK, Dickey CA, Baker BJ. Natural products as a rich source of tau-targeting drugs for Alzheimer’s disease. Future Med Chem. 2012;4:1751–61. https://doi.org/10.4155/FMC.12.124.
Hole KL, Williams RJ. Flavonoids as an intervention for Alzheimer’s disease: progress and hurdles towards defining a mechanism of action. Brain Plast. 2020;6:167–92. https://doi.org/10.3233/BPL-200098.
McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–97. https://doi.org/10.1007/S00401-013-1177-7.
Maccioni RB, Rojo LE, Fernández JA, Kuljis RO. The role of neuroimmunomodulation in Alzheimer’s disease. Ann N. Y Acad Sci. 2009;1153:240–6. https://doi.org/10.1111/J.1749-6632.2008.03972.X.
Morales I, Farías G, MacCioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17:202–4. https://doi.org/10.1159/000258724.
Maccioni RB, González A, Andrade V, Cortés N, Tapia JP, Guzmán-Martínez L. Alzheimer´s disease in the perspective of neuroimmunology. Open Neurol J. 2018;12:50–6. https://doi.org/10.2174/1874205X01812010050.
Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ). J Neuroinflammation. 2011;8:79. https://doi.org/10.1186/1742-2094-8-79.
Long HZ, Zhou ZW, Cheng Y, Luo HY, Li FJ, Xu SG, et al. The role of microglia in Alzheimer’s disease from the perspective of immune inflammation and iron metabolism. Front Aging Neurosci. 2022;14:888989. https://doi.org/10.3389/FNAGI.2022.888989.
Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy - Drug Targets. 2009;8:229–35.
Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–15. https://doi.org/10.1016/j.ejmech.2017.09.001.
Tunon M, Garcia-Mediavilla M, Sanchez-Campos S, Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–71. https://doi.org/10.2174/138920009787846369.
Choi S-M, Kim BC, Cho Y-H, Choi K-H, Chang J, Park M-S, et al. Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J. 2014;50:45–51. https://doi.org/10.4068/cmj.2014.50.2.45.
Wright JS. Predicting the antioxidant activity of curcumin and curcuminoids. J Mol Struct (Theochem). 2002;591:207–17. https://doi.org/10.1016/S0166-1280(02)00242-7.
Hao D-C. Drug metabolism and disposition diversity of ranunculales phytometabolites. In: Ranunculales Medicinal Plants. Elsevier. 2019;175–221. https://doi.org/10.1016/b978-0-12-814232-5.00005-8.
Vijayakumar BG, Ramesh D, Joji A, Jayachandra Prakasan J, Kannan T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020;886. https://doi.org/10.1016/J.EJPHAR.2020.173448.
Mizuno M, Mori K, Misawa T, Takaki T, Demizu Y, Shibanuma M, et al. Inhibition of β-amyloid–induced neurotoxicity by planar analogues of procyanidin B3. Bioorg Med Chem Lett. 2019;29:2659–63. https://doi.org/10.1016/j.bmcl.2019.07.038.
Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, et al. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003;36:1613–20. https://doi.org/10.1590/S0100-879X2003001200002.
Pinheiro RGR, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, et al. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur J Pharm Sci. 2020;148:105314. https://doi.org/10.1016/j.ejps.2020.105314.
Ferri P, Angelino D, Gennari L, Benedetti S, Ambrogini P, Del Grande P, et al. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2015;6:394–400. https://doi.org/10.1039/c4fo00817k.
Wei BB, Liu MY, Zhong X, Yao WF, Wei MJ. Increased BBB permeability contributes to EGCG-caused cognitive function improvement in natural aging rats: pharmacokinetic and distribution analyses. Acta Pharmacol Sin. 2019;40:1490–500. https://doi.org/10.1038/s41401-019-0243-7.
Zhao L, Wang JL, Liu R, Li XX, Li JF, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–65. https://doi.org/10.3390/MOLECULES18089949.
Fu X, Zhang J, Guo L, Xu Y, Sun L, Wang S, et al. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharm Biochem Behav. 2014;126:122–30. https://doi.org/10.1016/J.PBB.2014.09.005.
Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, et al. Protective effects of kaempferol (3,4′,5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci Biotechnol Biochem. 2010;74:397–401. https://doi.org/10.1271/BBB.90585.
Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO. Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol Neurobiol. 2017;54:2269–85. https://doi.org/10.1007/S12035-016-9795-4.
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–45. https://doi.org/10.1016/J.NEUROPHARM.2015.01.027.
Ramezani M, Darbandi N, Khodagholi F, Hashemi A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen Res. 2016;11:1976–80. https://doi.org/10.4103/1673-5374.197141.
Inoue T, Saito S, Tanaka M, Yamakage H, Kusakabe T, Shimatsu A, et al. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2019;116:10031–8. https://doi.org/10.1073/PNAS.1901659116.
Sun P, Yin JB, Liu LH, Guo J, Wang SH, Qu CH, et al. Protective role of dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci Rep. 2019;39:20180902. https://doi.org/10.1042/BSR20180902/195.
Cox CJ, Choudhry F, Peacey E, Perkinton MS, Richardson JC, Howlett DR, et al. Dietary (−)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiol Aging. 2015;36:178–87. https://doi.org/10.1016/J.NEUROBIOLAGING.2014.07.032.
Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–87. https://doi.org/10.1016/J.BRAINRES.2008.02.107.
Senolytic Therapy to Modulate Progression of Alzheimer’s Disease ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04063124.
Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD) Study ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04685590.
Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer´s Disease ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00951834.
Prevention of Cognitive Decline in ApoE4 Carriers With Subjective Cognitive Decline After EGCG and a Multimodal Intervention ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03978052.
COcoa Supplement and Multivitamin Outcomes Study for the Mind ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03035201.
Polyphenols and Risk of Dementia ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03286608.
Acknowledgements
MSD acknowledges Swami Vivekananda Merit-cum-means Scholarship scheme of Government of West Bengal for his fellowship. The author gratefully acknowledges Dr. Soumalee Basu of Department of Microbiology of University of Calcutta for sharing her knowledge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, M.S. A study from structural insight to the antiamyloidogenic and antioxidant activities of flavonoids: scaffold for future therapeutics of Alzheimer’s disease. Med Chem Res 32, 15–31 (2023). https://doi.org/10.1007/s00044-022-02990-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02990-z