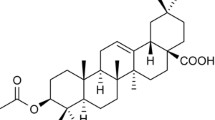
Abstract
Non-alcoholic fatty liver disease (NAFLD) is a prevalent chronic liver disease associated with hepatic lipid accumulation, insulin resistance, oxidative stress, and inflammation. The CFLAR-JNK pathway plays a decisive role in the development of NAFLD. EJ1 is a new hexacyclic triterpenic acid isolated from Euscaphis japonica, a traditional herbal medicine with anti-NAFLD effect. The present study was aimed to evaluate the hepatoprotective effect of EJ1 against NAFLD in vitro and the underlying mechanisms. The biochemical parameters related to lipid accumulation, insulin resistance, oxidative stress, and inflammation in oleic acid-induced HepG2 cellular model of NAFLD were measured. In this study, EJ1 was found to significantly decrease TG and TC contents. Meanwhile, EJ1 increased hepatocellular glucose uptake and improved insulin resistance by increasing the phosphorylation levels of pIRS1, pAKT and PI3K. Furthermore, EJ1 decreased the intracellular ROS and MDA, promoted the antioxidant enzymes activity including SOD and GSH-Px. Moreover, EJ1 reduced the levels of cellular NO and mRNA of TNF-α, IL-6 and IL-8. Further investigation showed that EJ1 treatment led to up-regulation of CFLAR protein expression and down-regulation of JNK protein phosphorylation. These results revealed that EJ1 has the potential to alleviate lipid accumulation, insulin resistance, oxidative stress, and inflammatory responses in OA-treated HepG2 cellular model of NAFLD, representing a promising leading compound for NAFLD, possibly by activating the CFLAR-JNK pathway.

Graphical abstract









Similar content being viewed by others
References
Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, Rao PN. The riddle of nonalcoholic fatty liver disease: progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. J Clin Exp Hepatol. 2015;5:147–58. https://doi.org/10.1016/j.jceh.2015.02.002.
Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–64. https://doi.org/10.1053/j.gastro.2020.01.052.
Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–71. https://doi.org/10.1002/hep.29724.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. https://doi.org/10.1016/s0016-5085(98)70599-2.
Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–41. https://doi.org/10.1007/s00535-013-0758-5.
Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:439–49. https://doi.org/10.1038/nm.4290.
Liu Y, Yu Q, Chen Y. Effect of silibinin on CFLAR-JNK pathway in oleic acid-treated HepG2 cells. Biomed Pharmacother. 2018;108:716–23. https://doi.org/10.1016/j.biopha.2018.09.089.
Ge YZ. Resources and utilization of EuscaphisJ japonica. Chin Wild Plant Resour. 2004;23:24–5.
Kim KH, Choi SH, Lee TS, Oh WK, Kim DS, Kim JB. Selective LXRalpha inhibitory effects observed in plant extracts of MEH184 (Parthenocissua tricuspidata) and MEH185 (Euscaphis japonica). Biochem Biophys Res Commun. 2006;349:513–8. https://doi.org/10.1016/j.bbrc.2006.08.092.
Lee MK, Jeon HY, Lee KY, Kim SH, Ma CJ, Sung SH, et al. Inhibitory constituents of Euscaphis japonica on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Med. 2007;73:782–6. https://doi.org/10.1055/s-2007-981551.
Lee MK, Lee KY, Jeon HY, Sung SH, Kim YC. Antifibrotic activity of triterpenoids from the aerial parts of Euscaphis japonica on hepatic stellate cells. J Enzym Inhib Med Chem. 2009;24:1276–9. https://doi.org/10.3109/14756360902829709.
Li YC, Tian K, Sun LJ, Long H, Li LJ, Wu ZZ. A new hexacyclic triterpene acid from the roots of Euscaphis japonica and its inhibitory activity on triglyceride accumulation. Fitoterapia. 2016;109:261–5. https://doi.org/10.1016/j.fitote.2016.01.016.
Li S, Liao X, Meng F, Wang Y, Sun Z, Guo F, et al. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS ONE. 2014;9:e86724. https://doi.org/10.1371/journal.pone.0086724.
Lin YN, Chang HY, Wang CCN, Chu FY, Shen HY, Chen CJ, et al. Oleanolic acid inhibits liver x receptor alpha and pregnane x receptor to attenuate ligand-induced lipogenesis. J Agric Food Chem. 2018;66:10964–76. https://doi.org/10.1021/acs.jafc.8b03372.
Toppo E, Sylvester Darvin S, Esakkimuthu S, Buvanesvaragurunathan K, Ajeesh Krishna TP, Antony Caesar S, et al. Curative effect of arjunolic acid from Terminalia arjuna in non-alcoholic fatty liver disease models. Biomed Pharmacother. 2018;107:979–88. https://doi.org/10.1016/j.biopha.2018.08.019.
Liou CJ, Dai YW, Wang CL, Fang LW, Huang WC. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019;33:11791–803. https://doi.org/10.1096/fj.201900413RRR.
Mu Q, Wang H, Tong L, Fang Q, Xiang M, Han L, et al. Betulinic acid improves nonalcoholic fatty liver disease through YY1/FAS signaling pathway. FASEB J. 2020;34:13033–48. https://doi.org/10.1096/fj.202000546R.
Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–8. https://doi.org/10.1007/s12272-009-1801-1.
Triquell MF, Diaz-Lujan C, Romanini MC, Ramirez JC, Paglini-Oliva P, Schijman AG, et al. Nitric oxide synthase and oxidative-nitrosative stress play a key role in placental infection by Trypanosoma cruzi. Am J Reprod Immunol. 2018;80:e12852. https://doi.org/10.1111/aji.12852.
Uysal S, Armutcu F, Aydogan T, Akin K, Ikizek M, Yigitoglu MR. Some inflammatory cytokine levels, iron metabolism and oxidan stress markers in subjects with nonalcoholic steatohepatitis. Clin Biochem. 2011;44:1375–9. https://doi.org/10.1016/j.clinbiochem.2011.09.017.
Xu JY, Zhang L, Li ZP, Ji G. Natural products on nonalcoholic fatty liver disease. Curr Drug Targets. 2015;16:1347–55. https://doi.org/10.2174/1389450116666150531155711.
Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010;2:95–104.
Perla FM, Prelati M, Lavorato M, Visicchio D, Anania C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children. 2017;4. https://doi.org/10.3390/children4060046.
Fabbrini E, Magkos F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients. 2015;7:4995–5019. https://doi.org/10.3390/nu7064995.
Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. https://doi.org/10.1002/hep.20920.
Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–75. https://doi.org/10.1053/j.gastro.2008.01.075.
Salamone F, Bugianesi E. Nonalcoholic fatty liver disease: the hepatic trigger of the metabolic syndrome. J Hepatol. 2010;53:1146–7. https://doi.org/10.1016/j.jhep.2010.06.013.
Zhang Y, Hai J, Cao M, Zhang Y, Pei S, Wang J, et al. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol. 2013;17:714–20. https://doi.org/10.1016/j.intimp.2013.08.019.
Gao Y, Zhang M, Zhang R, You L, Li T, Liu RH. Whole grain brown rice extrudate ameliorates the symptoms of diabetes by activating the IRS1/PI3K/AKT insulin pathway in db/db mice. J Agric Food Chem. 2019;67:11657–64. https://doi.org/10.1021/acs.jafc.9b04684.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. https://doi.org/10.1038/414799a.
Finck BN. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018;67:2485–93. https://doi.org/10.2337/dbi18-0024.
Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19:291–302. https://doi.org/10.1016/j.numecd.2008.12.015.
Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. https://doi.org/10.1155/2018/9547613.
Ntheazarian AR, Kangari P, Salmaninejad A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev. 2014;15:4745–51. https://doi.org/10.7314/apjcp.2014.15.12.4745.
Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. https://doi.org/10.1016/j.freeradbiomed.2011.10.003.
Chen J, Tian J, Ge H, Liu R, Xiao J. Effects of tetramethylpyrazine from Chinese black vinegar on antioxidant and hypolipidemia activities in HepG2 cells. Food Chem Toxicol. 2017;109:930–40. https://doi.org/10.1016/j.fct.2016.12.017.
Gehrke N, Schattenberg JM. Metabolic inflammation-A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158:1929–47 e6. https://doi.org/10.1053/j.gastro.2020.02.020.
Acknowledgements
This work was financially supported by the Program for Basic research project (free inquiry) of Shenzhen Science and Technology Plan (grant number JCYJ20170306171157738); National Natural Science Foundation of China (grant number 81560703); Open Foundation for National & Local Joint Engineering Research Center of High-throughput Drug Screening Technology (grant number M20181007, M20202003); and College Student Innovation and Entrepreneurship Training Program (grant number S202010512064).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Zhang, S., Lu, Y. et al. Effect of a hexacyclic triterpenic acid from Euscaphis japonica on the oleic acid induced HepG2 cellular model of non-alcoholic fatty liver disease. Med Chem Res 31, 2209–2219 (2022). https://doi.org/10.1007/s00044-022-02982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02982-z