Abstract
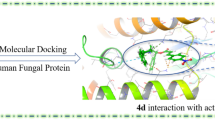
Some new N-substituted hetero aromatic compounds, derived from clotrimazole, were synthesized. In this regard, imidazole ring of clotrimazole was replaced by pyrazole (Series A; P1-P4) and benzimidazole moieties (Series B; P5-P8). All new compounds were evaluated against different species of fungi using broth microdilution method as recommended by clinical and laboratory standard institute (CLSI). Their cytotoxicity was assessed against MRC-5 as normal human fibroblasts cell line using MTT method. To augury the binding mode of the synthesized compounds against cytochrome P450 lanosterol 14α-demethylase, molecular docking studies were also performed. Our results indicated that some compounds showed desirable antifungal activities at concentrations ranging from 0.5 to 1 µg/mL. Among them, compounds 4-nitro-1-trityl-1H-pyrazole (P2) and 5-cyclopropyl-2-trityl-2H-pyrazol-3-ylamine (P4) which bearing pyrazole ring, had the most antifungal activities (MIC50 = 0.25–16 µg/mL), against all tested fungi species. Moreover, compound P4 showed bactericidal activity at higher concentration of MIC. In vitro cytotoxic evaluation revealed that the potent compounds (P2 and P4) were non-toxic at therapeutic dosages toward human cells. In addition, the results showed good correlation between docking energies and biological activities of the compounds. According to both antifungal and computational studies, P4, which containing special chemical structure, had desire potential to be consider as antifungal agent. Also, Density functional theory (DFT) was employed to study the reactivity descriptors of P4 such as HOMO-LUMO energy gap, electronegativity, electron affinity, ionization potential, molecular hardness, and molecular softness. Based on the DFT study, the heterocyclic residue of P4 has the favourable potent in accepting electrophilic reactions which is in agreement with the experimental data.

Graphical abstract






Similar content being viewed by others
References
Sperry JB, Wright DL. Furans, thiophenes and related heterocycles in drug discovery. Curr Opin drug Discov Dev. 2005;8:723–40.
Zamani L, Mirjalili BBF, Namazian M. One-pot synthesis of 2, 4, 5-trisubstituted-1H-imidazoles promoted by nano-TiCl. Chemija. 2013;24:312–9.
Zamani L, Mirjailli BBF, Zomorodian K, Zomorodian S. Synthesis and Characterization of 5-Substituted 1 H-Tetrazoles in the Presence of Nano-TiCl 4. SiO2. South Afr J Chem. 2015;68:133–7.
Emami L, Khabnadideh S, Faghih Z, Solhjoo A, Malek S, Mohammadian A, et al. Novel N-Substituted Isatin-Ampyrone Schiff Bases as a New Class of Antiproliferative Agents: Design, Synthesis, Molecular Modeling and in Vitro Cytotoxic Activity. J. Heterocyclic Chem. 2022;59:1144–59.
Abd-El Gawad NM, Georgey HH, Ibrahim NA, Amin NH, Abdelsalam RM. Synthesis of novel pyrazole and dihydropyrazoles derivatives as potential anti-inflammatory and analgesic agents. Arch pharmacal Res. 2012;35:807–21.
Abunada NM, Hassaneen HM, Samaha AS, Miqdad OA. Synthesis and antimicrobial evaluation of some new pyrazole, pyrazoline and chromeno [3, 4-c] pyrazole derivatives. J Braz Chem Soc. 2009;20:975–87.
Abunada NM, Hassaneen HM, Kandile NG, Miqdad OA. Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo [3, 4-d]-pyrimidine and pyrazolo [4, 3-e][1, 2, 4]-triazolo [1, 5-c] pyrimidine derivatives. Molecules. 2008;13:1501–17.
Zamani L, Mirjalili BBF, Zomorodian K, Namazian M, Khabnadideh S, Faghih Mirzaei E. Synthesis of benzimidazoles in the presence of nano-TiCl4. SiO2 as antifungal agents and tautomerism theoretical study of some products. Farmacia. 2014;62:3.
Zomorodian K, Khabnadideh S, Zamani L, Pakshir K, Tajaddod M. Evaluation of antifungal and antibacterial activity of some new benzimidazole derivatives. Lat Am Res Rev. 2018;48:125–9.
Alagarsamy V, Saravanan G. Synthesis and anticonvulsant activity of novel quinazolin-4 (3H)-one derived pyrazole analogs. Medicinal Chem Res. 2013;22:1711–22.
Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Gambari R, Bianchi N, et al. Synthesis, cytotoxicity, antitumor activity and sequence selective binding of two pyrazole analogs structurally related to the antitumor agents U-71,184 and adozelesin. Anti-Cancer Drug Des. 1997;12:555–76.
Gaba M, Mohan C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: recent advances and future directions. Medicinal Chem Res. 2016;25:173–210.
Lamb D, Kelly D, Kelly S. Molecular aspects of azole antifungal action and resistance. Drug Resistance Updates. 1999;2:390–402.
Chimenti F, Bizzarri B, Maccioni E, Secci D, Bolasco A, Fioravanti R, et al. Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg Medicinal Chem Lett. 2007;17:4635–40.
Crowley P, Gallagher H. Clotrimazole as a pharmaceutical: past, present and future. J Appl Microbiol. 2014;117:611–7.
Khabnadideh S, Rezaei Z, Khalafinezhad A, Pakshir K, Heiran MJ, Shobeiri H. Design and synthesis of 2-methyl and 2-methyl-4-nitro imidazole derivatives as antifungal agents. Iran J Pharm Sci. 2009;5:8.
Faghih Z, Rezaei Z, Jamshidzade A, Keshavarz A, Khabnadideh S. Cytotoxic Activity of Some Azole Derivatives. Asian Pac J Cancer Biol. 2018;3:79–82.
Faghih Z, Rezaei Z, Jamshidzadeh A, Faghih Z, Heidari N, Khabnadideh S. Cytotoxic evaluation of some new and potent azole derivatives as antimicrobial agents. Trends Pharm Sci. 2017;3:143–8.
Khabnadideh S, Harper JB. Effect of an Ionic liquid [Bmim][Tf2N] on the rate of reaction in the synthesis of some azole compounds as antifungal agents. Sci Res. 2014;2:125–31.
Zamani L, Faghih Z, Zomorodian K, Mirjalili BBF, Jalilian A, Khabnadideh S. Nano-SnCl4. SiO2, an efficient catalyst for synthesis of benzimidazole drivatives as antifungal and cytotoxic agents. Res Pharm Sci. 2019;14:496.
Gholami A, Khabnadideh S, Ghasemi Y, Mirjalili BBF, Shahmoradi R, Zamani L. TiCl4/nano-sawdust as an Efficient Biocatalyst for the Synthesis of Highly Substituted Dihydro-2-oxopyrroles as Antimicrobial Agents. J. Pharmaceutical Res. Int. 2017;16:1–14.
Khabnadideh S, Faghih Z, Zamani L, Zomorodian K, Mirjalili BBF, Moradi H. Nano-SnCl4/SiO2 as a Catalyst for One-Pot Synthesis of Substituted 1H-Pyrazoles as Antifungal and Cytotoxic Agents. Lett Org Chem. 2020;17:1-.
Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J medicinal Chem. 2009;44:3064–7.
Khabnadideh S, Rezaei Z, Pakshir K, Zomorodian K, Ghafari N. Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives. Res Pharm Sci. 2012;7:65.
Djuidje EN, Durini E, Sciabica S, Serra E, Balzarini J, Liekens S, et al. Skin damages—Structure activity relationship of benzimidazole derivatives bearing a 5-membered ring system. Molecules. 2020;25:4324.
Qiao L, Zhai ZW, Cai PP, Tan CX, Weng JQ, Han L, et al. Synthesis, crystal structure, antifungal activity, and docking study of difluoromethyl pyrazole derivatives. J Heterocycl Chem. 2019;56:2536–41.
Zinad DS, Mahal A, Shareef OA, editors. Antifungal activity and theoretical study of synthesized pyrazole-imidazole hybrids. IOP Conference Series: Materials Science and Engineering; 2020: IOP Publishing.
Hof H. Critical annotations to the use of azole antifungals for plant protection. Antimicrobial agents Chemother. 2001;45:2987–90.
Ghaedi M, Yousefi-Nejad M, Safarpoor M, Hashemi S, Goudarzi A, Tyagi I, et al. Investigation of phytochemical and antimicrobial properties of Linum usitatissimum in presence of ZnO/Zn (OH) 2 nanoparticles and extraction of euphol from Euphorbia microsciadia. Desalination Water Treat. 2016;57:20597–607.
Fereidoonnezhad M, Mirsadeghi HA, Abedanzadeh S, Yazdani A, Alamdarlou A, Babaghasabha M, et al. Synthesis and biological evaluation of thiolate gold (i) complexes as thioredoxin reductase (TrxR) and glutathione reductase (GR) inhibitors. N. J Chem. 2019;43:13173–82.
Emami L, Faghih Z, Sakhteman A, Rezaei Z, Faghih Z, Salehi F, et al. Design, synthesis, molecular simulation, and biological activities of novel quinazolinone-pyrimidine hybrid derivatives as dipeptidyl peptidase-4 inhibitors and anticancer agents. N. J Chem. 2020;44:19515–31.
Emami L, Faghih Z, Khabnadideh S, Rezaei Z, Sabet R, Harigh E, et al. 2-(Chloromethyl)-3-phenylquinazolin-4 (3H)-ones as potent anticancer agents; cytotoxicity, molecular docking and in silico studies. J. Iranian Chem.Society. 2021:1–13.
Yu TT, Kuppusamy R, Yasir M, Hassan M, Alghalayini A, Gadde S, et al. Design, Synthesis and Biological Evaluation of Biphenylglyoxamide-Based Small Molecular Antimicrobial Peptide Mimics as Antibacterial Agents. Int J Mol Sci. 2020;21:6789.
Köfeler HC, Fauler G, Windischhofer W, Leis HJ. Effect of cytochrome P‐450 inhibitors econazole, bifonazole and clotrimazole on prostanoid formation. Br J Pharmacol. 2000;130:1241–6.
Warrilow AG, Melo N, Martel CM, Parker JE, Nes WD, Kelly SL, et al. Expression, purification, and characterization of Aspergillus fumigatus sterol 14-α demethylase (CYP51) isoenzymes A and B. Antimicrobial agents Chemother. 2010;54:4225–34.
Bernhardt A, Meyer W, Rickerts V, Aebischer T, Tintelnot K. Identification of 14-α-lanosterol demethylase (CYP51) in scedosporium species. Antimicrobial agents Chemother. 2018;62:e02599–17.
Song JL, Harry JB, Eastman RT, Oliver BG, White TC. The Candida albicans lanosterol 14-α-demethylase (ERG11) gene promoter is maximally induced after prolonged growth with antifungal drugs. Antimicrobial agents Chemother. 2004;48:1136–44.
Emami L, Zamani L, Sabet R, Zomorodian K, Rezaei Z, Faghih Z, et al. Molecular Docking and Antimicrobial Evaluation of Some Novel Pyrano [2, 3-C] Pyrazole Derivatives. Trends Pharm Sci. 2020;6:113–20.
Morris GM, Huey R, Olson AJ. Using autodock for ligand‐receptor docking. Curr Protoc Bioinforma. 2008;24:8.14. 1-8. 40
Li Z, Gu J, Zhuang H, Kang L, Zhao X, Guo Q. Adaptive molecular docking method based on information entropy genetic algorithm. Appl Soft Comput. 2015;26:299–302.
Becke A. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648.
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785.
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, et al. Gaussian 09, rev. Gaussian Inc, Wallingford. 2009.
Mirhendi H, Makimura K, Zomorodian K, Maeda N, Ohshima T, Yamaguchi H. Differentiation of Candida albicans and Candida dubliniensis using a single-enzyme PCR-RFLP method. Jpn J Infect Dis. 2005;58:235.
Wayne P. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A2. 2002.
Hashemi S, Jassbi AR, Erfani N, Kiani R, Seradj H. Two new cytotoxic ursane triterpenoids from the aerial parts of Salvia urmiensis Bunge. Fitoterapia 2021;154:105030.
Hashemi S, Seradj H, Kiani R, Jassbi AR, Erfani N Salvurmin A and et al., Two Ursane Triterpenoids of Salvia Urmiensis Induce Apoptosis and Cell Cycle Arrest in Human Lung Carcinoma Cells. Pharmaceutical Sci. 2022. https://doi.org/10.34172/PS.2022.32.
Yadav A, Yadav M, Kumar S, Yadav JP. Bactericidal effect of Acacia nilotica: In Vitro antibacterial and time kill kinetic studies. Int J Curr Res. 2015;7:22289–94.
Akhlaghi S, Mostoufi A, Kalantar H, Fereidoonnezhad M. Synthesis and biological evaluations of novel pyrazinoic acid derivatives as anticancer agents. Medicinal Chem Res. 2022;31:580–93.
Sadeghian S, Emami L, Mojaddami A, Faghih Z, Zomorodian K, Rashidi M, et al. 1, 2, 4-Triazole derivatives as novel and potent antifungal agents: Design, synthesis and biological evaluation. J. Mol. Struct. 2022;1271:134039–51.
Acknowledgements
Financial assistance from the Shiraz University of Medical Sciences by way of grant number 93-01-05-7697 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emami, L., Faghih, Z., Zomorodian, K. et al. Clotrimazole-based hybrid structures of pyrazole and benzimidazole: synthesis, antifungal evaluation and computational studies. Med Chem Res 31, 2220–2230 (2022). https://doi.org/10.1007/s00044-022-02981-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02981-0