Abstract
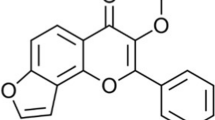
Alzheimer’s disease (AD) is one of the chronic neurodegenerative pathologies that lead to memory loss and mental and behavioral changes in elderly people. The senile plaques, neurofibrillary tangles, oxidative stress, increased acetylcholinesterase (AChE), and neuroinflammation by activating 5-lipoxygenases (5-LOX) are important pathological processes in AD. Phyllanthus niruri Linn (PN) earned a lot of attention for phytoconstituents and their medicinal properties. The compounds quercitrin and niruriflavone were isolated by bio-guided fractionation from PN using the in vitro assays. Both compounds showed good docking scores on AChE and 5-LOX targets in the molecular docking studies. AD was induced in rats by 100 mg/kg of oral aluminum chloride (AlCl3) for 42 days. It decreased the antioxidative enzymes and increased lipid peroxidation and AChE activity. Oral administration of niruriflavone reversed the neurobehavioral changes caused by AlCl3. The niruriflavone treatment also restored the antioxidative enzymes and attenuated the AChE and lipid peroxidation. All the evidence suggests that isolated compounds could benefit the population afflicted by AD in a multitargeted manner.

Graphical abstract





Similar content being viewed by others
References
Wang X, Wang W, Li L, Perry G, Lee H, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2014;1842:1240–7.
Carvajal FJ, Inestrosa NC. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: therapeutic relevance of IDN 5706. Front Mol Neurosci. 2011;4:19 https://doi.org/10.3389/fnmol.2011.00019.
Garcia-Ayllon MS, Small DH, Avila J, Saez-Valero J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: crosstalk with P-tau and β-amyloid. Front Mol Neurosci. 2011;4:1–9.
Dzoyem JP, Eloff JN. Anti-inflammatory, anticholinesterase, and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J Ethnopharmacol. 2015;160:194–201. https://doi.org/10.1016/j.jep.2014.11.034.
Joshi Y, Pratico D. Neuroinflammation and Alzheimer’s disease: lessons learned from 5-lipoxygenase. Transl Neurosci. 2014;5:197–202. https://doi.org/10.2478/s13380-014-0225-7.
Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M. Alzheimer’s Disease International. World Alzheimer report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. https://www.alz.co.uk/research/world-report-2015.
Nichols E. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet. 2022;7:E105–25.
Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397.
Kim MJ, Rehman SU, Amin FU, Kim MO. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomedicine. 2017;13:2533 https://doi.org/10.1016/j.nano.2017.06.022.
Abdel-Aal RA, Assi AA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol. 2011;659:169–76. https://doi.org/10.1016/j.ejphar.2011.03.011. PMID: 21440537.
Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharm. 2006;58:1559–70. https://doi.org/10.1211/jpp.58.12.0001. PMID: 17331318.
Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A. et al. Effect of Phyllanthus niruri treatment on liver, kidney, and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008;46:514–20.
Anuar N, Markom M, Khairedin S, Johari N. A production and extraction of quercetin and (+)-catechin from Phyllanthus niruri callus culture. Int J Biol Biomol. 2012;6:968–71.
Sharma P, Parmar J, Verma P, Sharma P, Goyal PK. Anti-tumor activity of Phyllanthus niruri (a medicinal plant) on chemical-induced skin carcinogenesis in mice. Asian Pac J Cancer Prev. 2009;10:1089–94. PMID: 20192590.
Hardiyanti R, Marpaung L, Adnyana K, Simanjuntak P. Isolation of quercitrin from Dendrophthoe pentandra (l.) miq leaves and its antioxidant and antibacterial activities. Rasayan J Chem. 2019;12:1822–27.
Utari F, Itam A, Syafrizayanti S, Putri WH, Ninomiya M, Koketsu M. et al. Isolation of flavonol rhamnosides from Pometia pinnata leaves and investigation of α-glucosidase inhibitory activity of flavonol derivatives. J Appl Pharm Sci. 2019;9:53–65.
Than NN, Fotso S, Poeggeler B, Hardeland R, Laatsch H. Niruriflavone, a new antioxidant flavone sulfonic acid from Phyllanthus niruri. Z für Na turforschung B. 2006;61:57–60. https://doi.org/10.1515/znb-2006-0111.
Andrianto D, Widianti W, Bintang M. Antioxidant and cytotoxic activity of Phyllanthus acidus fruit extracts. IOP Conf Ser Earth Environ Sci. 2017;58:1–5. https://doi.org/10.1088/1755-1315/58/1/012022.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Disco. 2021;20:689–709. https://doi.org/10.1038/s41573-021-00233-1.
Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011:809696. https://doi.org/10.1155/2011/809696.
Mossa AT, Nawwar GA. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum Exp Toxicol. 2011;30:1501–13. https://doi.org/10.1177/0960327110391686. PMID: 21239482.
Lin CZ, Zhu CC, Hu M, Wu AZ, Bairu ZD, Kangsa SQ. Structure-activity relationships of antioxidant activity in vitro about flavonoids isolated from Pyrethrum tatsienense. J Intercult Ethnopharmacol. 2014;3:123–7. https://doi.org/10.5455/jice.20140619030232.
Noreen H, Semmar N, Farman M, McCullagh JSO. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac J Trop Med. 2017;10:792–801. https://doi.org/10.1016/j.apjtm.2017.07.024. PMID: 28942828.
Joshi YB, Domenico P. The 5-lipoxygenase pathway: oxidative and inflammatory contributions to the Alzheimer’s disease phenotype. Front Mol Neurosci. 2015;8:436. https://doi.org/10.3389/fncel.2014.00436.
Gomes A, Fernandes E, Lima JL, Mira L, Corvo ML. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr Med Chem. 2008;15:1586–605. https://doi.org/10.2174/092986708784911579. PMID: 18673226.
Alhawarri MB, Dianita R, Razak KNA, Mohamad S, Nogawa T, Wahab HA. Antioxidant, anti-inflammatory, and inhibition of acetylcholinesterase potentials of Cassia timoriensis DC. flowers. Molecules. 2021;26:2594 https://doi.org/10.3390/molecules26092594. PMID: 33946788; PMCID: PMC8125573.
Nam G, Hong M, Lee J, Lee HJ, Ji Y, Kang J. et al. Multiple reactivities of flavonoids towards pathological elements in Alzheimer’s disease: structure–activity relationship. Chem Sci. 2020;37:10243–54.
Cavallaro V, Braun AE, Ravelo AG, Murray AP. Sulphated flavonoid isolated from Flaveria bidentis and its semisynthetic derivatives as potential drugs for Alzheimer’s disease. In Proceedings of the 17th International Electronic Conference on Synthetic Organic Chemistry. Basel, Switzerland: MDPI; 2013. https://doi.org/10.3390/ecsoc-17-b011.
Figueira I, Garcia G, Pimpão RC, Terrasso AP, Costa I, Almeida AF. et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci Rep. 2017;7:11456. https://doi.org/10.1038/s41598-017-11512-6.
Shailima R, Mary RL. Network based approach in the establishment of the relationship between type 2 diabetes mellitus and its complications at the molecular level coupled with molecular docking mechanism. Biomed Res Int. 2016;2016:6068437. https://doi.org/10.1155/2016/6068437.
Bhuvanendran S, Hanapi NA, Ahemad N, Othman I, Yusof SR, Shaikh MF. Embelin, a potent molecule for Alzheimer’s disease: a proof of concept from blood-brain barrier permeability, acetylcholinesterase inhibition and molecular docking studies. Front Neurosci. 2019;13:495 https://doi.org/10.3389/fnins.2019.00495.
Singh NA, Bhardwaj V, Ravi C, Ramesh N, Mandal AKA, Khan ZA. EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat model of Alzheimer’s disease. Front Aging Neurosci. 2018;10:244. https://doi.org/10.3389/fnagi.2018.00244.
Thippeswamy AH, Rafiq M, Viswantha GL, Kavya KJ, Anturlikar SD, Patki PS. Evaluation of Bacopa monniera for its synergistic activity with rivastigmine in reversing aluminum-induced memory loss and learning deficit in rats. J Acupunct Meridian Stud. 2013;6:208–13. https://doi.org/10.1016/j.jams.2013.02.004. PMID: 23972243.
Kumar A, Prakash A, Dogra S. Neuroprotective effect of carvedilol against aluminium induced toxicity: possible behavioral and biochemical alterations in rats. Pharm Rep. 2011;63:915–23. https://doi.org/10.1016/s1734-1140(11)70607-7. PMID: 22001979.
Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49:2906–13. https://doi.org/10.1016/j.fct.2011.08.006. PMID: 21889563.
Kaizer RR, Corrêa MC, Spanevello RM, Morsch VM, Mazzanti CM, Gonçalves JF. et al. Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem. 2005;99:1865–70. https://doi.org/10.1016/j.jinorgbio.2005.06.015. 16055195.
Kaur A, Gill KD. Possible peripheral markers for chronic aluminium toxicity in Wistar rats. Toxicol Ind Health. 2006;22:39–46. https://doi.org/10.1191/0748233706th242oa. PMID: 16572710.
Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci. 2021;28:4232–9. https://doi.org/10.1016/j.sjbs.2021.06.031.
Al-Mamary M, Al-Habori M, Al-Zubairi AS. The in vitro antioxidant activity of different types of palm dates (Phoenix dactylifera) syrups. Arab J Chem. 2014;7:964–71.
Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Alter Med. 2008;8:63 https://doi.org/10.1186/1472-6882-8-63.
Mathew M, Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS One. 2014;9:e86804 https://doi.org/10.1371/journal.pone.0086804.
Chung LY, Soo WK, Chan KY, Mustafa MR, Goh SH, Imiyabir Z. Lipoxygenase inhibiting activity of some Malaysian plants. Pharm Biol. 2009;47:1142–48. https://doi.org/10.3109/13880200903008724.
Ishola AA, Oyinloye BE, Basiru A, Kappo AP. Molecular docking studies of flavonoids from Andrographis paniculata as potential acetylcholinesterase, butyrylcholinesterase and monoamine oxidase inhibitors towards the treatment of neurodegenerative diseases. Biointerface Res Appl Chem. 2020;11:9871–9. https://doi.org/10.33263/BRIAC113.98719879.
Yadavalli R, Peasari JR, Mamindla P, Praveenkumar, Mounika S, Ganugapati J. Phytochemical screening and in silico studies of flavonoids from Chlorella pyrenoidosa. Inf Med Unlocked. 2018;10:89–99.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60.
Bhalla P, Garg ML, Dhawan DK. Protective role of lithium during aluminium-induced neurotoxicity. Neurochem Int. 2010;56:256–62. https://doi.org/10.1016/j.neuint.2009.10.009.
Prakash A, Kumar A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin Pharm Toxicol. 2009;105:98e104.
Cheng L, Pan GF, Sun XB, Huang YX, Peng YS, Zhou LY. Evaluation of anxiolytic-like effect of aqueous extract of asparagus stem in mice. Evid Based Complement Altern Med. 2013;2013:587260 https://doi.org/10.1155/2013/587260. PMID: 24348707; PMCID: PMC3853311.
Ellman GL, Courtney KD, Andres V Jr, feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm. 1961;7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9. PMID: 13726518.
Luck H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1971. p. 885–93.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:48670–7.
Kono Y. Generation of superoxide radical during auto-oxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95.
Wills ED. Mechanism of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajamanickam, G., SL, M. Bio-guided isolation of anti-Alzheimer’s compounds from Phyllanthus niruri and role of niruriflavone in the reversal of aluminum chloride-induced neurobehavioral and biochemical changes in an animal model. Med Chem Res 31, 1740–1753 (2022). https://doi.org/10.1007/s00044-022-02944-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02944-5