Abstract
Prostacyclin (PGI2) is a natural hormone that has primarily been explored for its potent vasodilation activity and inhibition of platelet aggregation. The chemical structure of prostacyclin has intrinsic instability in aqueous solutions that limited its analysis and clinical use. Therefore, different analogues have been designed and synthesized with similar activity as prostacyclin and with greatly improved stability. Moreover, structural modifications in its α- and ω-sidechains for some analogues resulted in better activity and/or potency and other unique functions. This review covered the discovery and chemistry of prostacyclin. The current and emerging drugs with their structural scaffolds are also reviewed herein with a detailed comparison of their structural modifications and clinical effects.
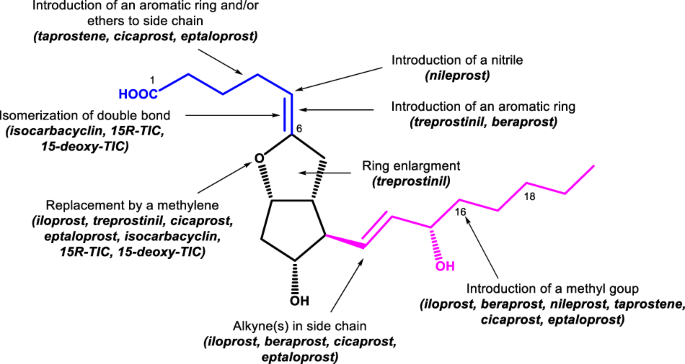
Graphical abstract












Similar content being viewed by others
References
Mitchell JA, Ahmetaj-Shala B, Kirkby NS, Wright WR, Mackenzie LS, Reed DM, et al. Role of prostacyclin in pulmonary hypertension. Glob Cardiol Sci Pr. 2014;2014:382–93. https://doi.org/10.5339/gcsp.2014.53
Zardi EM, Zardi DM, Dobrina A, Afeltra A. Prostacyclin in sepsis: a systematic review. Prostaglandins Other Lipid Mediat. 2007;83:1–24. https://doi.org/10.1016/j.prostaglandins.2006.12.004
Rahman MS. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J Cell Physiol. 2019;234:3254–62. https://doi.org/10.1002/jcp.26932
McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–7. https://doi.org/10.1073/pnas.96.1.272
Smyth EM, Austin SC, Reilly MP, FitzGerald GA. Internalization and sequestration of the human prostacyclin receptor. J Biol Chem. 2000;275:32037–45. https://doi.org/10.1074/jbc.M003873200
Bolego C, Buccellati C, Prada A, Gaion RM, Folco G, Sala A. Critical role of COX-1 in prostacyclin production by human endothelial cells under modification of hydroperoxide tone. FASEB J. 2009;23:605–12. https://doi.org/10.1096/fj.08-106591
Piplani P, Aggarwal D, Abbhi V, Saini L. Prostaglandin analogues: current treatment option for glaucoma. Med Chem Res. 2016;25:1031–48. https://doi.org/10.1007/s00044-016-1563-5
Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharm. 2019;176:1038–50. https://doi.org/10.1111/bph.14167
Granström E. Prostaglandin chemistry. Acta Obstet et Gynecol Scand. 1979;58:13–4. https://doi.org/10.3109/00016347909157783
Clapp LH, Gurung R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015;120:56–71. https://doi.org/10.1016/j.prostaglandins.2015.04.007
Lee K, Lee SH, Kim TH. The biology of prostaglandins and their role as a target for allergic airway disease therapy. Int J Mol Sci. 2020;21:1–26. https://doi.org/10.3390/ijms21051851
Prasher P, Mudila H, Sharma M, Khati B. Developmental perspectives of the drugs targeting enzyme-instigated inflammation: a mini review. Med Chem Res. 2019;28:417–49. https://doi.org/10.1007/s00044-019-02315-7
Boswell MG, Zhou W, Newcomb DC, Peebles RS Jr. PGI2 as a regulator of CD4+ subset differentiation and function. Prostaglandins Other Lipid Mediat. 2011;96:21–6. https://doi.org/10.1016/j.prostaglandins.2011.08.003
Di Costanzo F, Di Dato V, Ianora A, Romano G. Prostaglandins in marine organisms: A review. Mar Drugs. 2019;17:17 https://doi.org/10.3390/md17070428
Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–5. https://doi.org/10.1038/263663a0
Moncada S, Gryglewski RJ, Bunting S, Vane JR. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976;12:715–37. https://doi.org/10.1016/0090-6980(76)90048-4
Johnson RA, Morton DR, Kinner JH, Gorman RR, McGuire JC, Sun FF, et al. The chemical structure of prostaglandin X (prostacyclin). Prostaglandins. 1976;12:915–28. https://doi.org/10.1016/0090-6980(76)90126-X
Gryglewski RJ. Prostacyclin among prostanoids. Pharm Rep. 2008;60:3–11.
Vane J, Corin RE. Prostacyclin: a vascular mediator. Eur J Vasc Endovasc Surg. 2003;26:571–8. https://doi.org/10.1016/s1078-5884(03)00385-x
Moncada S, Korbut R, Bunting S, Vane JR. Prostacyclin is a circulating hormone. Nature. 1978;273:767–8. https://doi.org/10.1038/273767a0
Moncada S, Vane JR. Prostacyclin: Its biosynthesis, actions and clinical potential. Philos Trans R Soc Lond B Biol Sci. 1981;294:305–29. https://doi.org/10.1098/rstb.1981.0108
Arehart E, Gleim S, Kasza Z, Fetalvero KM, Martin KA, Hwa J. Prostacyclin, atherothrombosis, and cardiovascular disease. Curr Med Chem. 2007;14:2161–9. https://doi.org/10.2174/092986707781389637
Awad I, Little JR, Lucas F, Skrinska V, Slugg R, Lesser RP. Treatment of acute focal cerebral ischemia with prostacyclin. Stroke. 1983;14:203–9. https://doi.org/10.1161/01.STR.14.2.203
Stitham J, Arehart EJ, Gleim SR, Douville KL, Hwa J. Human prostacyclin receptor structure and function from naturally-occurring and synthetic mutations. Prostaglandins Other Lipid Mediat. 2007;82:95–108. https://doi.org/10.1016/j.prostaglandins.2006.05.010
Stitham J, Midgett C, Martin KA, Hwa J. Prostacyclin: An inflammatory paradox. Front Pharm. 2011;2:24 https://doi.org/10.3389/fphar.2011.00024
Olschewski H, Rose F, Schermuly R, Ghofrani HA, Enke B, Olschewski A, et al. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharm Ther. 2004;102:139–53. https://doi.org/10.1016/j.pharmthera.2004.01.003
Reid HM, Kinsella BT. Prostacyclin receptors: Transcriptional regulation and novel signaling mechanisms. Prostaglandins Other Lipid Mediat. 2015;121:70–82. https://doi.org/10.1016/j.prostaglandins.2015.04.008
Del Pozo R, Hernandez Gonzalez I, Escribano-Subias P. The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev Respir Med. 2017;11:491–503. https://doi.org/10.1080/17476348.2017.1317599
Sasaki Y, Kuwata H, Akatsu M, Yamakawa Y, Ochiai T, Yoda E, et al. Involvement of prostacyclin synthase in high-fat-diet-induced obesity. Prostaglandins Other Lipid Mediat. 2021;153:106523 https://doi.org/10.1016/j.prostaglandins.2020.106523
Scheeren T, Radermacher P. Prostacyclin (PGI2): new aspects of an old substance in the treatment of critically ill patients. Intensive Care Med. 1997;23:146–58. https://doi.org/10.1007/s001340050309
Orekhov A, Tertov V, Smirnov V. Prostacyclin analogues as antiatherosclerotic drugs. Lancet. 1983;322:521 https://doi.org/10.1016/S0140-6736(83)90555-X
Schneider MR, Tang DG, Schirner M, Honn KV. Prostacyclin and its analogues: antimetastatic effects and mechanisms of action. Cancer Metast Rev. 1994;13:349–64. https://doi.org/10.1007/BF00666104
Fetalvero KM, Martin KA, Hwa J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. 2007;82:109–18. https://doi.org/10.1016/j.prostaglandins.2006.05.011
Paramothayan NS, Lasserson TJ, Wells AU, Walters EH Prostacyclin for pulmonary hypertension in adults. Cochrane Database Syst Rev 2005;:CD002994. https://doi.org/10.1002/14651858.CD002994.pub2
Safdar Z. Treatment of pulmonary arterial hypertension: The role of prostacyclin and prostaglandin analogs. Respir Med. 2011;105:818–27. https://doi.org/10.1016/j.rmed.2010.12.018
Pluchart H, Khouri C, Blaise S, Roustit M, Cracowski JL. Targeting the prostacyclin pathway: beyond pulmonary arterial ypertension. Trends Pharm Sci. 2017;38:512–23. https://doi.org/10.1016/j.tips.2017.03.003
Chen S-H, Chen L-K, Teng T-H, Chou WH. Comparison of inhaled nitric oxide with aerosolized prostacyclin or analogues for the postoperative management of pulmonary hypertension: a systematic review and meta-analysis. Ann Med. 2020;52:120–30. https://doi.org/10.1080/07853890.2020.1746826
Mandras SA, Mehta HS, Vaidya A. Pulmonary hypertension: A brief guide for clinicians. Mayo Clin Proc. 2020;95:1978–88. https://doi.org/10.1016/j.mayocp.2020.04.039. 2020;95:1978–88
Hansmann G, Sallmon H, Roehr CC, Kourembanas S, Austin ED, Koestenberger M, et al. Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr Res. 2021;89:446–55. https://doi.org/10.1038/s41390-020-0993-4
Cristo Ropero MJ, Cruz-Utrilla A, Escribano-Subias MP. Epoprostenol for the treatment of pulmonary arterial hypertension. Expert Rev Clin Pharm. 2021;14:1005–13. https://doi.org/10.1080/17512433.2021.1929925
Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A. A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet. 2016;55:1495–505. https://doi.org/10.1007/s40262-016-0409-0
Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901. https://doi.org/10.1183/09031936.00097107
Abu Deiab GI, Croatt MP. Synthetic approaches to isocarbacyclin and analogues as potential neuroprotective agents against ischemic stroke. Bioorg Med Chem. 2019;27:338–42. https://doi.org/10.1016/j.bmc.2018.12.010
Abu Deiab GI, Croatt MP Chapter 3 - Step-economical synthesis of clinprost and analogs utilizing a novel decarboxylation reaction. In: Harmata M, editor. Strategies and Tactics in Organic Synthesis. Academic Press; 2016. p. 95–117. https://doi.org/10.1016/B978-0-08-100756-3.00003-0
Capra V, Bäck M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev. 2013;33:364–438. https://doi.org/10.1002/med.21251
Cho MJ, Allen MA. Chemical stability of prostacyclin (PGI2) in aqueous solutions. Prostaglandins. 1978;15:943–54. https://doi.org/10.1016/0090-6980(78)90037-0
Rosenkranz B, Fischer C, Frölich JC. Prostacyclin metabolites in human plasma. Clin Pharm Ther. 1981;29:420–4. https://doi.org/10.1038/clpt.1981.58
Deshpande SP, Mazzeffi MA, Strauss E, Hollis A, Tanaka KA. Prostacyclins in cardiac surgery: Coming of age. Semin Cardiothorac Vasc Anesth. 2018;22:306–23. https://doi.org/10.1177/1089253217749298
Scholz H. Prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;285:R512–514. https://doi.org/10.1152/ajpregu.00298.2003
Wu KK, Liou J-Y. Cellular and molecular biology of prostacyclin synthase. Biochem Biophys Res Commun. 2005;338:45–52. https://doi.org/10.1016/j.bbrc.2005.08.021
Chiang C-W, Yeh H-C, Wang L-H, Chan NL. Crystal structure of the human prostacyclin synthase. J Mol Bio. 2006;364:266–74. https://doi.org/10.1016/j.jmb.2006.09.039
Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med. 2010;104:9–21. https://doi.org/10.1016/j.rmed.2009.07.015
Honorato Pérez J. Selexipag, a selective prostacyclin receptor agonist in pulmonary arterial hypertension: a pharmacology review. Expert Rev Clin Pharm. 2017;10:753–62. https://doi.org/10.1080/17512433.2017.1322900
Brown MM, Pickles H. Effect of epoprostenol (prostacyclin, PGI2) on cerebral blood flow in man. J Neurol Neurosurg Psychiatry. 1982;45:1033–6. https://doi.org/10.1136/jnnp.45.11.1033
Mikhailidis DP, Mikhailidis AM, Barradas MA, Dandona P. Infusion of prostacyclin (epoprostenol). Lancet. 1982;2:767 https://doi.org/10.1016/s0140-6736(82)90949-7
Barnes H, Yeoh H-L, Fothergill T, Burns A, Humbert M, Williams T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2019;5:CD012785 https://doi.org/10.1002/14651858.CD012785.pub2
Stubbe B, Opitz CF, Halank M, Halank M, Habedank D, Ewert R. Intravenous prostacyclin-analogue therapy in pulmonary arterial hypertension - A review of the past, present and future. Respir Med. 2021;179:106336 https://doi.org/10.1016/j.rmed.2021.106336
Gais H-J, Kramp GJ, Wolters D, Reddy LR. Development of a common fully stereocontrolled access to the medicinally important and promising prostacyclin analogues iloprost, 3-oxa-iloprost and cicaprost. Chem Eur J. 2006;12:5610–7. https://doi.org/10.1002/chem.200600187
Baker SE, Hockman RH. Inhaled iloprost in pulmonary arterial hypertension. Ann Pharmacother. 2005;39:1265–74. https://doi.org/10.1345/aph.1E575
Chen Y, Shi J, Li L, Liu F, Zhang X, Yang Y. An alternative synthesis for iloprost via a key bicyclic aldehyde intermediate. Tetrahedron Lett. 2021;62:152627 https://doi.org/10.1016/j.tetlet.2020.152627
Mereles D, Ewert R, Lodziewski S, Borst MM, Benz A, Olschewski H, et al. Effect of inhaled iloprost during off-medication time in patients with pulmonary arterial hypertension. Respiration. 2007;74:498–502. https://doi.org/10.1159/000101953
Piazza G, Creager MA. Thromboangiitis obliterans. Circ. 2010;121:1858–61. https://doi.org/10.1161/CIRCULATIONAHA.110.942383
Cacione DG, Macedo CR, do Carmo Novaes F, Baptista-Silva JC. Pharmacological treatment for Buerger’s disease. Cochrane Database Syst Rev. 2020;5:CD011033 https://doi.org/10.1002/14651858.CD011033.pub4
Matsumura K, Watanabe Y, Onoe H, Watanabe Y. Prostacyclin receptor in the brain and central terminals of the primary sensory neurons: an autoradiographic study using a stable prostacyclin analogue [3H]iloprost. Neuroscience. 1995;65:493–503. https://doi.org/10.1016/0306-4522(94)00505-y
El Yafawi R, Wirth JA. What is the role of oral prostacyclin pathway medications in pulmonary arterial hypertension management? Curr Hypertens Rep. 2017;19:97 https://doi.org/10.1007/s11906-017-0796-0
McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, et al. Efficacy and safety of treprostinil: An epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharm. 2003;41:293–9.
Feldman J, Habib N, Fann J, Radosevich JJ. Treprostinil in the treatment of pulmonary arterial hypertension. Future Cardiol. 2020;16:547–58. https://doi.org/10.2217/fca-2020-0021
Corboz MR, Salvail W, Gagnon S, LaSala D, Laurent CE, Salvail D, et al. Prostanoid receptor subtypes involved in treprostinil-mediated vasodilation of rat pulmonary arteries and in treprostinil-mediated inhibition of collagen gene expression of human lung fibroblasts. Prostaglandins Other Lipid Mediat. 2021;152:106486 https://doi.org/10.1016/j.prostaglandins.2020.106486
Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N. Engl J Med. 2021;384:325–34. https://doi.org/10.1056/NEJMoa2008470
Patterson JH, Adams KF Jr, Gheorghiade M, Bourge RC, Sueta CA, Clarke SW, et al. Acute hemodynamic effects of the prostacyclin analog 15AU81 in severe congestive heart failure. Am J Cardiol. 1995;75:26A–33A. https://doi.org/10.1016/s0002-9149(99)80380-4
Guigui A, Mazet R, Blaise S, Cracowski C, Beau-Guillaumot M, Kotzki S, et al. Treprostinil hydrogel iontophoresis in systemic sclerosis-related digital skin ulcers: A safety study. J Clin Pharm. 2020;60:758–67. https://doi.org/10.1002/jcph.1574
Roustit M, Gaillard-Bigot F, Blaise S, Stanke-Labesque F, Cracowski C, Seinturier C, et al. Cutaneous iontophoresis of treprostinil in systemic sclerosis: A proof-of-concept study. Clin Pharm Ther. 2014;95:439–45. https://doi.org/10.1038/clpt.2013.255
García-Lacuna J, Domínguez G, Blanco-Urgoiti J, Pérez-Castells J. Synthesis of treprostinil: key Claisen rearrangement and catalytic Pauson–Khand reactions in continuous flow. Org Biomol Chem. 2019;17:9489–501. https://doi.org/10.1039/C9OB02124H
Barst RJ, McGoon M, McLaughlin V, Tapson V, Oudiz R, Shapiro S, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119–25. https://doi.org/10.1016/S0735-1097(03)00463-7
Nowrouzi-Sohrabi P, Tabrizi R, Hessami K, Shabani-Borujeni M, Hosseini-Bensenjan M, Rezaei S, et al. The effects of beraprost sodium on renal function and cardiometabolic profile in patients with diabetes mellitus: a systematic review and meta-analysis of clinical trials. Int Urol Nephrol. 2022;54:111–20. https://doi.org/10.1007/s11255-021-02887-7
Umemiya S, Sakamoto D, Kawauchi G, Hayashi Y. Enantioselective total synthesis of beraprost using organocatalyst. Org Lett. 2017;19:1112–5. https://doi.org/10.1021/acs.orglett.7b00134
Higuchi K, Sawada K, Nambu H, Shogaki T, Kita Y. A convenient synthesis of the beraprost intermediate: A useful method for introducing a C3 unit at the benzyl position. Org Lett. 2003;5:3703–4. https://doi.org/10.1021/ol035371
Yoneda M, Ohkawa Y. Degradation and isomerization of nileprost, 5-cyano-16-methyl-prostacyclin, in aqueous solution. Chem Pharm Bull. 1990;38:2507–12. https://doi.org/10.1248/cpb.38.2507
Takahashi A, Kirio Y, Sodeoka M, Sasai H, Shibasaki M. Highly stereoselective synthesis of exocyclic tetrasubstituted enol ethers and olefins. A synthesis of nileprost. J Am Chem Soc. 1989;111:643–7. https://doi.org/10.1021/ja00184a036
Krause W, Nieuweboer B. Development of a radioimmunoassay and its application to the pharmacokinetics of the stable prostacyclin analogue, nileprost, in the rat. Prostaglandins Leukot Med. 1984;14:1–9. https://doi.org/10.1016/0262-1746(84)90017-9
Darius H, Thomsen T, Senior K. Cardiovascular actions in vitro and cardioprotective effects in vivo of nileprost, a mixed type PGI2/PGE2 agonist. J Cardiovasc Pharm. 1987;10:144–52.
Bergman NA, Halvarsson T. Chemical stability of a prostacyclin analog due to the absence of intramolecular catalysis. J Org Chem. 1988;53:2548–52. https://doi.org/10.1021/jo00246a027
Chiang Y, Kresge AJ, Seipp U, Winter W. Kinetics of hydrolysis of vinyl ether functional group of the stable, bioactive prostacyclin analog taprostene (CG 4203). J Org Chem. 1988;53:2552–5. https://doi.org/10.1021/jo00246a028
Virgolini I, Fitscha P, Sinzinger H, Barth H. Effects of taprostene, a stable prostacyclin analogue, on haemodynamics, platelet function and arachidonate metabolism in healthy volunteers. Eur J Clin Pharm. 1990;38:347–50. https://doi.org/10.1007/BF00315573
Michel G, Seipp U. In vitro studies with the stabilized epoprostenol analogue taprostene. Effect on platelets and erythrocytes. Arzneimittelforschung. 1990;40:817–22.
Schneider J. Beneficial effects of the prostacyclin analogue taprostene on cardiovascular, pulmonary and renal disturbances in endotoxin-shocked rabbits. Eicosanoids. 1991;4:99–105.
Robertson L, Andras A Prostanoids for intermittent claudication. Cochrane Database Syst Rev 2013;:CD000986. https://doi.org/10.1002/14651858.CD000986.pub3
Vitale V, Monami M, Mannucci E. Prostanoids in patients with peripheral arterial disease: A meta-analysis of placebo-controlled randomized clinical trials. J Diabetes Complications. 2016;30:161–6. https://doi.org/10.1016/j.jdiacomp.2015.09.006
Chan K, Jones RL. Partial agonism of taprostene at prostanoid IP receptors in vascular preparations from Guinea-pig, rat, and mouse. J Cardiovasc Pharm. 2004;43:795–807.
Jones RL, Chan KM. Investigation of the agonist activity of prostacyclin analogues on prostanoid EP4 receptors using GW 627368 and taprostene: evidence for species differences. Prostaglandins Leukot Ess Fat Acids. 2005;72:289–99. https://doi.org/10.1016/j.plefa.2004.12.002
Schneider MR, Schirner M, Lichtner RB, Graf H. Antimetastatic action of the prostacyclin analogue cicaprost in experimental mammary tumors. Breast Cancer Res Tr. 1996;38:133–41. https://doi.org/10.1007/BF01803791
Lerm M, Gais H-J, Cheng K, Vermeeren C. Asymmetric synthesis of the highly potent anti-metastatic prostacyclin analogue cicaprost and its isomer isocicaprost. J Am Chem Soc. 2003;125:9653–67. https://doi.org/10.1021/ja030200l
Corey EJ, Helal CJ. An efficient catalytic stereoselective route to a key intermediate for the synthesis of the long-lived PGI2 analog ZK 96480 (CicaprostTM). Tetrahedron Lett. 1997;38:7511–4. https://doi.org/10.1016/S0040-4039(97)01803-0
Hildebrand M. Inter-species extrapolation of pharmacokinetic data of three prostacyclin-mimetics. Prostaglandins. 1994;48:297–312. https://doi.org/10.1016/0090-6980(94)90030-2
Ossenkamp KL, Gais H-J. Total synthesis of (+)-3-oxacarbacyclin 2. stereoselective deprotonation and completion of the synthesis. Liebigs Ann. 1997;1997:2433–41. https://doi.org/10.1002/jlac.199719971206
Hildebrand M. Bioactivation of eptaloprost in animals and man. Prostaglandins. 1993;46:177–89. https://doi.org/10.1016/0090-6980(93)90043-7
Aiken JW, Shebuski RJ. Comparison in anesthetized dogs of the anti-aggregatory and hemodynamic effects of prostacyclin and a chemically stable prostacyclin analog, 6a-carba-PGI2 (carbacyclin). Prostaglandins. 1980;19:629–43. https://doi.org/10.1016/s0090-6980(80)80011-6
Whittle BJ, Moncada S, Whiting F, Vane JR. Carbacyclin - a potent stable prostacyclin analogue for the inhibition of platelet aggregation. Prostaglandins. 1980;19:605–27. https://doi.org/10.1016/s0090-6980(80)80010-4
Adaikan PG, Lau LC, Tai MY, Karim SMM. Inhibition of platelet aggregation with intravenous and oral administration of a carboprostacyclin analogue, 15-cyclopentyl-ω-pentanor-5(E)-carbacyclin (ONO 41483) in man. Prostaglandins Leukot Med. 1983;10:53–64. https://doi.org/10.1016/S0262-1746(83)80020-1
Nagy EE, Hyatt IF, Gettys KE, Yeazell ST, Frempong SK Jr, Croatt MP. Sequential Pd(0)-, Rh(I)-, and Ru(II)-catalyzed reactions in a nine-step synthesis of clinprost. Org Lett. 2013;15:586–9. https://doi.org/10.1021/ol303402e
Tanaka M, Kojima C, Muramatsu M, Tanabe H. Binding affinities of isocarbacyclin methyl ester and its free acid to prostanoid receptors. Arzneimittelforschung. 1995;45:967–70.
Sheddan NA, Mulzer J. Exploration of omega-side chain addition strategies for the syntheses of isocarbacyclin and 15R-16-(m-tolyl)-17,18,19,20-tetranorisocarbacyclin. Org Biomol Chem. 2006;4:4127–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abu Deiab, G.I., Croatt, M.P. Prostacyclin (PGI2) scaffolds in medicinal chemistry: current and emerging drugs. Med Chem Res 31, 1241–1251 (2022). https://doi.org/10.1007/s00044-022-02914-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02914-x




