Abstract
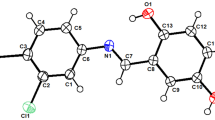
Bioactive molecules displaying visible wavelength emission can be useful for bioimaging, chemosensing and photodynamic therapy applications. Reported herein are 1,3,4-trisubsituted-1,2,3-triazolium salts displaying both antimicrobial and visible emission properties. Using a click chemistry approach, 2-fluorenyl, 1-naphthyl, 2-naphthyl, 2-anthracenyl and 1-pyrenyl units were incorporated at the N1 position, imparting visible emission properties to their triazolium bromide salts with Stokes shifts greater than 100 nm relative to the emission of their triazole precursors. The increasing size of such hydrophobic aryl units impacts minimum inhibitory concentration (MIC) values against Gram-positive bacteria, Gram-negative bacteria and yeast, and can be counterbalanced by hydrophobic substituent variation at other positions of the molecule in order to preserve bioactivity. Among the series of compounds studied are analogs displaying blue, green and yellow colored emission and MIC values as low as 0.4 μM (Gram-positive bacteria), 8 μM (Gram-negative bacteria) and 2 μM (yeast). XRD analysis validates the regioselective benzylation at the N3 position of the 1,2,3-triazole ring and the ability of such compounds to associate through dimeric intermolecular π-stacking interactions.

Graphical Abstract







Similar content being viewed by others
References
US Department of Health and Human Services, CDC. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention. 2019:1-113. https://doi.org/10.15620/cdc:82532.
WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) report 2021. WHO; 2021. https://www.who.int/publications/i/item/9789240027336.
Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health?. Microb Drug Resist.2010;16:91–104. https://doi.org/10.1089/mdr.2009.0120.
Hora PI, Pati SG, McNamara PJ, Arnold WA. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7:622–31. https://doi.org/10.1021/acs.estlett.0c00437.
Jennings MC, Minbiole KPC, Wuest WM. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect Dis. 2016;1:288–303. https://doi.org/10.1021/acsinfecdis.5b00047.
Tezel U, Pavlostathis SG. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol. 2015;33:296–304. https://doi.org/10.1016/j.copbio.2015.03.018.
Wales AD, Davies RH. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4:567–604. https://doi.org/10.3390/antibiotics4040567.
Minbiole KPC, Jennings MC, Ator LE, et al. From antimicrobial activity to mechanism of resistance: the multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron. 2016;72:3559–66. https://doi.org/10.1016/j.tet.2016.01.014.
Jennings MC, Forman ME, Duggan SM, Minbiole KPC, Wuest WM. Efflux pumps might not be the major drivers of QAC resistance in methicillin-resistant staphylococcus aureus. ChemBioChem. 2017;18:1573–7. https://doi.org/10.1002/cbic.201700233.
Alkhalifa S, Jennings MC, Granata D, et al. Analysis of the destabilization of bacterial membranes by quaternary ammonium compounds: a combined experimental and computational study. ChemBioChem. 2020;21:1510–6. https://doi.org/10.1002/cbic.201900698.
Vereshchagin AN, Frolov NA, Egorova KS, Seitkalieva MM, Ananikov VP. Quaternary ammonium compounds (Qacs) and ionic liquids (ils) as biocides: from simple antiseptics to tunable antimicrobials. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22136793
Carden RG, Sommers KJ, Schrank CL, et al. Advancements in the development of non-nitrogen-based amphiphilic antiseptics to overcome pathogenic bacterial resistance. ChemMedChem. 2020;15:1974–84. https://doi.org/10.1002/cmdc.202000612.
Fletcher JT, Sobczyk JM, Gwazdacz SC, Blanck AJ. Antimicrobial 1,3,4-trisubstituted-1,2,3-triazolium salts. Bioorg Med Chem Lett. 2018;28:3320–3. https://doi.org/10.1016/j.bmcl.2018.09.011.
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–9. https://doi.org/10.1002/1521-3773.
Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–64. https://doi.org/10.1021/jo011148j.
Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–21. https://doi.org/10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.3.co;2-x.
Meldal M, Tornøe CW. Cu-catalyzed azide-alkyne cycloaddition. Chem Rev. 2008;108:2952–3015. https://doi.org/10.1021/cr0783479.
Perera HA, Yan M. Imaging, identification and inhibition of microorganisms using AIEgens. Top Curr Chem. 2021;379:1–24. https://doi.org/10.1007/s41061-021-00333-x.
Stone MRL, Butler MS, Phetsang W, Cooper MA, Blaskovich MAT. Fluorescent antibiotics: new research tools to fight antibiotic resistance. Trends Biotechnol. 2018;36:523–36. https://doi.org/10.1016/j.tibtech.2018.01.004.
Zhao C, Fernandez A, Avlonitis N, et al. Searching for the optimal fluorophore to label antimicrobial peptides. ACS Comb Sci. 2016;18:689–96. https://doi.org/10.1021/acscombsci.6b00081.
Fletcher JT, Keeney ME, Walz SE. 1-Allyl- and 1-benzyl-3-methyl-1,2,3-triazolium salts via tandem click transformations. Synthesis. 2010;19:3339–45. https://doi.org/10.1055/s-0030-1257909.
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition. CLSI document M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015.
CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—third edition. CLSI document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
American Society for Microbiology. Manual of antimicrobial susceptibility testing.; 2005. https://doi.org/10.1007/s13398-014-0173-7.2.
Duhamel J. New insights in the study of pyrene excimer fluorescence to characterize macromolecules and their supramolecular assemblies in solution. Langmuir. 2012;28:6527–38. https://doi.org/10.1021/la2047646.
Winnik F. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem Rev. 1993;93:587–614. https://doi.org/10.1021/cr00018a001.
Cao Q-Y, Pradhan T, Lee MH, No K, Kim JS. Ferrocene-based anion receptor bearing amide and triazolium donor groups. Analyst. 2012;137:4454. https://doi.org/10.1039/c2an35481k.
Li Y, Zhao Y, Jiang R, Liu H, Li Y. Synthesis of a naphthalenediimide-based cyclophane for controlling anion-arene interactions. Inorg Chem Front. 2014;1:661–7. https://doi.org/10.1039/c4qi00095a.
Hoyer C, Schwerk P, Suntrup L, et al. Synthesis, characterization, and evaluation of antibacterial activity of ferrocenyl-1,2,3-triazoles, triazolium salts, and triazolylidene complexes of gold(i) and silver(i). Eur J Inorg Chem. 2021;2021:1373–82. https://doi.org/10.1002/ejic.202100024.
Lawal NS, Bala MD. Click synthesis and characterization of 1,2,3-triazolium salts. J Mol Struct. 2020;1200:127124. https://doi.org/10.1016/j.molstruc.2019.127124.
Mullen KM, Mercurio J, Serpell CJ, Beer PD. Exploiting the 1,2,3-triazolium motif in anion-templated formation of a bromide-selective rotaxane host assembly. Angew Chem Int Ed. 2009;48:4781–4. https://doi.org/10.1002/anie.200901313.
Chan PY, Kwok WM, Lam SK, Chiu P, Phillips DL. Time-resolved resonance raman observation of the 2-fluorenylnitrenium ion reaction with guanosine to form a C8 intermediate. J Am Chem Soc 2005;127:8246–7. https://doi.org/10.1021/ja0505651.
Hu M, Li J, Yao SQ. In situ “click” assembly of small molecule matrix metalloprotease inhibitors containing zinc-chelating groups. Org Lett. 2008;10:5529–31. https://doi.org/10.1021/ol802286g.
Boshev G, Dyall L, Sadler P. Pyrolysis of aryl azides. II. Naphthyl azides. Aust J Chem. 1972;25:599. https://doi.org/10.1071/CH9720599.
Xie F, Sivakumar K, Zeng Q, Bruckman MA, Hodges B, Wang Q. A fluorogenic “click” reaction of azidoanthracene derivatives. Tetrahedron. 2008;64:2906–14. https://doi.org/10.1016/j.tet.2008.01.080.
Brase S, Gil C, Knepper K, Zimmermann V. Organic azides: an exploding diversity of a unique class of compounds. Angew Chem Int Ed. 2005;44:5188–240. https://doi.org/10.1002/anie.200400657.
Rigaku Oxford Diffraction, CrysAlis PRO (2020), Oxford Diffraction /Agilent Technologies UK Ltd, Yarnton, England.
Sheldrick GM. SHELXT – integrated space-group and crystal-structure determination. Acta Cryst. 2015;A71:3–8. https://doi.org/10.1107/S2053273314026370.
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Cryst. 2015;C71:3–8. https://doi.org/10.1107/S2053229614024218.
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst. 2009;42:339–41. https://doi.org/10.1107/S0021889808042726.
Images and video generated using CrystalMaker®: a crystal and molecular structures program for Mac and Windows. CrystalMaker Software Ltd, Oxford, England (http://www.crystalmaker.com).
Acknowledgements
This publication was made possible by grants from the National Institute for General Medical Science (NIGMS) (5P20GM103427), a component of the National Institutes of Health (NIH), and its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lejcher, C.A., Villa, E.M. & Fletcher, J.T. Merging antimicrobial and visible emission properties within 1,3,4-trisubstituted-1,2,3-triazolium salts. Med Chem Res 31, 474–484 (2022). https://doi.org/10.1007/s00044-022-02855-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02855-5