Abstract
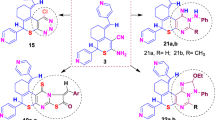
A series of 3-phosphonylated thiazolo[3,2-a]oxopyrimidines 3a-k was synthesized for the first time by the reactions of chloroethynylphosphonates with 5,6-disubstituted 2-thiouracils. In vitro antiviral activities have shown that the compounds 1i, 1j, 3b and 3e were shown activity against influenza A virus. In vitro antitumor activity was conducted for all compounds against human erythroleukemia (K562) and cervical carcinoma (HeLa) cell lines by MTS assay. Among targeted compounds 3c, 3h and 3j were active against human erythroleukemia (K562) cell line, while 3c and 3j were active against cervical carcinoma (HeLa) cell line. It was discovered that HeLa cells after treatment with compounds 3c and 3j significantly reduced the number of cells with stress fibers and with filopodium-like membrane protrusions. It was concluded that targeted compounds have a cytostatic effect, which could lead to a decrease in the formation of actin filaments and also in the number of filopodium-like membrane protrusions.









Similar content being viewed by others
References
Zhao L, Yan Y, Dai Q, Li X, Xu K, Zou G, et al. Development of novel anti -influenza thiazolides with 2 relatively broad-spectrum antiviral potentials.antimicrob. Agents Chemother. 2020;64:e00222–20. https://doi.org/10.1128/AAC.00222-20
Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Suzuki T, et al. Inhibition of HBV transcription from cccDNA with nitazoxanide by targeting the HBx–DDB1 interaction. Cell Mol Gastroenterol Hepatol. 2019;7:297–312. https://doi.org/10.1016/j.jcmgh.2018.10.010
Munir M, Rafique S, Ali A, Idrees M, Min Kang S. Calculation of wiener indices of thiazolides: the potent inhibitors of hepatitis B virus and hepatitis C virus replication. Hepat Mon. 2018;18:e67709. https://doi.org/10.5812/hepatmon.67709
Zuccaro V, Lombardi A, Asperges E, Sacchi P, Bruno R. PK/PD and antiviral activity of anti-HCV therapy: is there still a role in the choice of treatment? Expert Opin Drug Metab Toxicol. 2020;16:97–101. https://doi.org/10.1080/17425255.2020.1721459
Ozyilmaz E. Antiviral Agents. J Crit Intensive Care. 2020;11:27–29. https://doi.org/10.37678/dcybd.2020.2380
Blot N, Schneider P, Young P, Janvresse C, Dehesdin D, Tron P, et al. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transpl. 2000;26:903–5. https://doi.org/10.1038/sj.bmt.1702591.
Cornely OA, Hoenigl M. Infection Management in Hematology. Hematologic Malignancies. Cham: Springer; 2021.
El Haddad L, Ghantoji S, Park A, Batista M, Schelfhout J, Hachem J, et al. Clinical and economic burden of pre-emptive therapy of cytomegalovirus infection in hospitalized allogeneic hematopoietic cell transplant recipients. J Med Virol. 2020;92:86–95. https://doi.org/10.1002/jmv.25574
Han MS, Choi EH, Lee HJ, Yun KW, Kang HJ, Hong KT, et al. Cytomegalovirus disease in a retinoblastoma cohort: the role of preemptive screening. Pediatr Blood Cancer. 2019;67:e28101. https://doi.org/10.1002/pbc.28101
De Lima Moreira F, Marques MP, Duarte G, Lanchote VL. Determination of raltegravir and raltegravir glucuronide in human plasma and urine by LC–MS/MS with application in a maternal-fetal pharmacokinetic study. J Pharm Biomed Anal. 2020;117:112838. https://doi.org/10.1016/j.jpba.2019.112838
Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, Martin R. Infrequent development of resistance in genotype 1–6 hepatitis C virus–infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis. 2014;59:1666–74. https://doi.org/10.1093/cid/ciu697
Chuchkov K, Chayrov R, Hinkov A, Todorov D, Shishkova K, Stankova IG. Modifications on the heterocyclic base of ganciclovir, penciclovir, acyclovir - syntheses and antiviral properties. Nucleosides, Nucleotides Nucleic Acids. 2020;39:979–90. https://doi.org/10.1080/15257770.2020.1725043
Mohamed SF, Flefel EM, Amr AE-GE, Abd El-Shafy D. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur J Med Chem. 2010;45:1494–501. https://doi.org/10.1016/j.ejmech.2009.12.057
Fatima S, Sharma A, Saxena R, Tripathi R, Shukla SK, Pandey SK, et al. One pot efficient diversity oriented synthesis of polyfunctional styryl thiazolopyrimidines and their bio-evaluation as antimalarial and anti-HIV agents. Eur J Med Chem. 2012;55:195–204. https://doi.org/10.1016/j.ejmech.2012.07.018
Yaragatti NB, Kulkarni MV, Ghate MD, Hebbar SS, Hegde GR. Synthesis and biological evaluation of some new coumarinyl thiazolopyrimidinones. J Sulfur Chem. 2010;31:123–33. https://doi.org/10.1080/17415990903569544
Sayed M, Kamal El-Dean AM, Ahmed M, Hassanien R. Design, synthesis, and characterization of novel pyrimidines bearing indole as antimicrobial agents. J Chin Chem Soc. 2019;66:218–25. https://doi.org/10.1002/jccs.201800115
Morsy HA, Moustafa AH. A facile approach for synthesis of thiazines-, thiazoles- and triazoles-annulated 6-styryl-2-thiouracil derivative. J Iran Chem Soc. 2020;17:119–25. https://doi.org/10.1007/s13738-019-01760-w
Mohamed MM, Ali KKH, Eslam MA, AbeerM El-N. Design, synthesis of new pyrimidine derivatives as anticancer and antimicrobial agents. Synth Commun. 2017;47:1441–57. https://doi.org/10.1080/00397911.2017.1332223
El-Borai MA, Rizk HF, Ibrahim SA, El-Sayed HF. Microwave assisted synthesis of fused thiazoles in multicomponent system and their in vitro antitumor, antioxidant, and antimicrobial activities. J Heterocycl Chem. 2017;54:1031–41. https://doi.org/10.1002/jhet.2671
Hassan MZ, Alsayari A, Osman H, Ali MA, Muhsinah AB, Ahsan MJ. Synthesis, and evaluation of coumarin hybrids as antimycobacterial agents. ActaPoloniaePharmaceutica – Drug Res. 2019;76:1029–36. https://doi.org/10.32383/appdr/112406
Ramadan SK, El-Helw EAE, Sallam HA. Cytotoxic and antimicrobial activities of some novel heterocycles employing 6-(1,3-diphenyl1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4- tetrahydropyrimidine-5-carbonitrile. Heterocycl Commun. 2019;25:107–15. https://doi.org/10.1515/hc-2019-0008
Salem MS, Farhat M, Errayes AO, Madkour HM. Antioxidant activity of novel fused heterocyclic compounds derived from tetrahydropyrimidine derivative. Chem Pharm Bull. 2015;63:866–72. https://doi.org/10.1248/cpb.c15-00452
Christopherson RI, Lyons SD. Potent inhibitors of de novo pyrimidine and purine biosynthesis as chemotherapeutic agents. Med Res Rev. 1990;10:505–48. https://doi.org/10.1002/med.2610100406
Filatov AS, Knyazev NA, Shmakov SV, Bogdanov AA, Ryazantsev MN, Shtyrov AA, et al. Concise synthesis of tryptanthrin spiro analogues with in vitro antitumor activity based on one-pot, three-component 1,3-dipolar cycloaddition of azomethine ylides to сyclopropenes. Synthesis. 2019;51:713–29. https://doi.org/10.1055/s-0037-1611059.
Mirgayazova R, Khadiullina R, Mingaleeva R, Chasov V, Gomzikova M, Garanina E, et al. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol Biol Res Commun. 2019;8:119–28. https://doi.org/10.22099/mbrc.2019.34179.1419.
Wang S, Filatov AS, Lozovskiy SV, Shmakov SV, Khoroshilova OV, Larina AG, et al. Construction of Spiro[3-azabicyclo[3.1.0]hexanes] via 1,3-Dipolar Cycloaddition of 1,2-Diphenylcyclopropenes to Ninhydrin-Derived Azomethine Ylides. Synthesis. 2021;53:2114–32. https://doi.org/10.1055/a-1360-9716
Raffa D, Daidone G, Maggio B, Cascioferro S, Plescia F, Schillaci D. Synthesis and antileukemic activity of new 3-(5-methylisoxazol-3-yl) and 3-(pyrimidin-2-yl)-2-styrylquinazolin-4(3H)-ones. Il Farm. 2004;59:451–5. https://doi.org/10.1016/j.farmac.2003.10.006
Jeanneau-Nicolle E, Benoit-Guyod M, Namil A, Leclerc G. New thiazolo[3,2-a]pyrimidine derivatives, synthesis and structure-activity relationships. Eur J Med Chem. 1992;27:115–20. https://doi.org/10.1016/0223-5234(92)90099-M
Youssef MSK, Ahmed RA, Abbady MS, Abdel-Mohsen SA, Omar AA. Reactions of 4-(2-aminothiazole-4-yl)-3-methyl-5-oxo-1-phenyl-2-pyrazoline. Synthesis of thiazolo[3,2-a]pyrimidine and imidazo[2,1-b]thiazole derivatives. Monatsh Chem. 2008;139:553–9. https://doi.org/10.1007/s00706-007-0817-9
Veretennikov EA, Pavlov AV. Synthesis of 5H-[1,3]Thiazolo[3,2-a]pyrimidin-5-one Derivatives. Russ J Org Chem. 2013;49:575–9. https://doi.org/10.1134/s1070428013040143
Frolova TV, Kim DG, Sharutin VV, Shalkova EN. Synthesis and Heterocyclization of 2-{[2-(4-Bromophenyl)-2-oxoethyl]sulfanyl}pyrimidin-4(3H)-ones. Russ J Org Chem. 2016;52:96–98.
Balalaie S, Bararjanian M, Sheikh‐Ahmadi M, Hekmat S, Salehi P. Diammonium hydrogen phosphate: an efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Synth Commun. 2007;37:1097–108. https://doi.org/10.1080/00397910701196579
Krylov AS, Petrosian AA, Piterskaya JL, Svintsitskaya NI, Dogadina AV. Synthesis of ([1,2,4]triazolo[4,3-a]pyridin-3-ylmethyl)phosphonates and their benzo derivatives via 5-exo-dig cyclization. Beilstein J Org Chem. 2019;15:1563–8. https://doi.org/10.3762/bjoc.15.159.
Kidwai M, Saxena S, Mohan R, Venkataramanan R. A novel one pot synthesis of nitrogen containing heterocycles: an alternate methodology to the Biginelli and Hantzsch reactions. J Chem Soc Perkin Trans 1. 2002;1:1845–6. https://doi.org/10.1039/b205539m
Khudina OG, Ivanova AE, Burgart YV, Pervova MG, Shatunova TV, Borisevich SS, et al. Alkylation of 6-Polyfluoroalkyl-2-thiouracils with Haloalkanes. Russ J Org Chem. 2019;55:782–91. https://doi.org/10.1134/s1070428019060071
Ivanova AE, Khudina OG, Burgart YV, Pervova MG, Ezhikova MA, Kodess MI, et al. 6-Trifl uoromethyl-2-thiouracil and its analogs in reactions with 4-bromobutyl acetate and 2-bromoacetophenone. Russ Chem Bull. 2019;68:1190–5. https://doi.org/10.1007/s11172-019-2538-8.
Kaskevich KI, Babushkina AA, Gurzhiy VV, Egorov DM, Svintsitskaya NI, Dogadina AV. Synthesis of 3(2)-phosphonylated thiazolo[3,2-a]oxopyrimidines. Beilstein J Org Chem. 2020;16:1947–54. https://doi.org/10.3762/bjoc.16.161
Xu H, Zhang YI, Huang J, Chen W. Copper-catalyzed synthesis of N-fused heterocycles through regioselective 1,2-aminothiolation of 1,1-dibromoalkenes. Org Lett. 2010;12:3704–7. https://doi.org/10.1021/ol101563f
Egorov DM, Babushkina AA, Leonenok VE, Chekalov AP, Piterskaya Yu L. Synthesis of 3-phosphorylated thiazolo[3,2-a]pyrimidine-6-carboxylates. Russ J Gen Chem. 2020;90:326–8. https://doi.org/10.1134/S1070363220020267
Egorov DM, Piterskaya YL, Dogadina AV. Reaction of Chloroethynylphosphonates with 1-Methyl-3H-imidazole-2-thiones. Russ J Gen Chem. 2015;85:502–4. https://doi.org/10.1134/S1070363215020255
Tojkander S, Gateva G, Lappalainen P. Actin stress fibers – assembly, dynamics and biological roles. J Cell Sci. 2012;125:1855–64. https://doi.org/10.1242/jcs.098087
Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. https://doi.org/10.1038/ncb1019
Sun L, Zheng J, Wang Q, Song R, Liu H, Meng R, et al. NHERF1 regulates actin cytoskeleton organization through modulation of α-actinin-4 stability. FASEB J. 2016;30:578–89.
Knyazev NA, Shmakov SV, Pechkovskaya SA, Filatov AS, Stepakov AV, Boitsov VM, et al. Identification of spiro-fused [3-azabicyclo[3.1.0]hexane]oxindoles as potential antitumor agents: initial in vitro evaluation of anti-proliferative effect and actin cytoskeleton transformation in 3T3 and 3T3-SV40 fibroblast. Int J Mol Sci. 2021;22:8264. https://doi.org/10.3390/ijms22158264
Aseervatham J. Cytoskeletal remodeling in cancer. Biology. 2020;9:385. https://doi.org/10.3390/biology9110385
Spano A, Monaco G, Barni S, Sciola L. Cisplatin treatment of NIH/3T3 cultures induces a form of autophagic death in polyploid cells. Histol Histopathol. 2008;23:717–30. https://doi.org/10.14670/HH-23.717
Bonello TT, Stehn JR, Gunning PW. New approaches to targeting the actin cytoskeleton for chemotherapy. Future Med Chem. 2009;1:1311–31. https://doi.org/10.4155/fmc.09.99
Northcott JM, Dean IS, Mouw JK, Weaver VM. Feeling stress: the mechanics of cancer progression and aggression. Front Cell Dev Biol. 2018;6:17 https://doi.org/10.3389/fcell.2018.00017
Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. https://doi.org/10.1016/j.tcb.2010.08.015
Brayford S, Schevzov G, Vos J, Gunning P. The role of the actin cytoskeleton in cancer and its potential use as a therapeutic target. In: Schatten H, ed. The Cytoskeleton in Health and Disease. New York, NY: Springer New York; 2015. https://doi.org/10.1007/978-1-4939-2904-7_16.
Acknowledgements
We gratefully acknowledge the financial support from the Russian Foundation for Basic Research (Grant no. 19-03-00365) and the Ministry of Education and Science of the Russian Federation (0791-2020-0006). This research made use of resources from the Engineering Centre of Saint-Petersburg State Institute of Technology, the Centre for Chemical Analysis and Materials, and the Center for X-ray Diffraction Methods of Saint-Petersburg State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no cmpeting interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Babushkina, A.A., Dogadina, A.V., Egorov, D.M. et al. Efficient synthesis and evaluation of antiviral and antitumor activity of novel 3-phosphonylated thiazolo[3,2-a]oxopyrimidines. Med Chem Res 30, 2203–2215 (2021). https://doi.org/10.1007/s00044-021-02801-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02801-x