Abstract
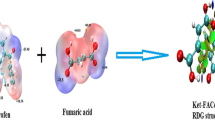
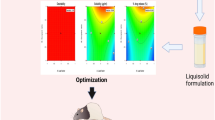
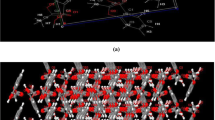
In the present study, ketoprofen-p-aminobenzoic acid (KP-PABA) co-crystal was prepared, to advance solubility and dissolution rate of drug, by solvent evaporation technique employing central composite experimental design. The optimized batch as recommended by the experimental design was characterized by FTIR, DSC, XRD, SEM, and NMR studies and further, evaluated for in-vitro and in-vivo anti-inflammatory and analgesic activities. The solubility and % drug release of different batches of co-crystal was found to be between 34.20–60.11 µg/ml and 68.11–93.45%, respectively. Physical characterization by X-ray diffraction spectra and differential scanning calorimetric studies confirmed the crystallinity of prepared co-crystal. The IC50 value of optimized batch of co-crystal formulation and pure drug was observed as 248.79 µg/ml and 524.40 µg/ml, respectively, displaying that co-crystal formulation possesses more percentage protection against protein denaturation than the drug ketoprofen. The results of in-vivo anti-inflammatory activity carried out by rat paw edema method revealed that the optimized batch of co-crystal preparation provided a significant % inhibition in paw volume in contrast to standard drug in wistar rats. Hence, the crystalline molecular complex of ketoprofen with p-aminobenzoic acid was documented that set out an improvement in solubility and also in anti-inflammatory activity of the drug in wistar rats.







Similar content being viewed by others
References
Sarraguça MC, Ribeiro PR, Dos Santos AO, Lopes JA. Batch statistical process monitoring approach to a cocrystallization process. J Pharm Sci. 2015;104:4099–108. https://doi.org/10.1002/jps.24623.
Soares FL, Carneiro RL. Green synthesis of ibuprofen–nicotinamide cocrystals and in-line evaluation by Raman spectroscopy. Cryst Growth Des. 2013;13:1510–7. https://doi.org/10.1021/cg3017112.
Harriss BI, Vella-Zarb L, Wilson C, Evans IR. Furosemide cocrystals: Structures, hydrogen bonding, and implications for properties. Cryst Growth Des. 2014;14:783–91. https://doi.org/10.1021/cg401662d.
Padrela L, Rodrigues MA, Velaga SP, Matos HA, de Azevedo EG. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur J Pharm Sci. 2009;38:9–17. https://doi.org/10.1016/j.ejps.2009.05.010.
Chow SF, Shi L, Ng WW, Leung KH, Nagapudi K, Sun CC, et al. Kinetic entrapment of a hidden curcumin cocrystal with phloroglucinol. Cryst Growth Des. 2014;14:5079–89. https://doi.org/10.1021/cg5007007.
Childs SL, Stahly GP, Park A. The salt− cocrystal continuum: the influence of crystal structure on ionization state. Mol Pharm. 2007;4:323–38. https://doi.org/10.1021/mp0601345.
Berry DJ, Steed JW. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv Drug Deliv Rev. 2017;117:3–24. https://doi.org/10.1016/j.addr.2017.03.003.
da Silva CC, Pepino RD, de Melo CC, Tenorio JC, Ellena J. Controlled synthesis of new 5-fluorocytosine cocrystals based on the p K a rule. Cryst Growth Des. 2014:4383–93. https://doi.org/10.1021/cg500502j.
Aitipamula S, Chow PS, Tan RB. Polymorphism in cocrystals: a review and assessment of its significance. Cryst Eng Comm. 2014;16:3451–65. https://doi.org/10.1039/C3CE42008F.
Lee KS, Kim KJ, Ulrich J. Formation of salicylic acid/4, 4′‐dipyridyl cocrystals based on the ternary phase diagram. Chem Eng Technol. 2015;38:1073–80. https://doi.org/10.1002/ceat.201400738.
Shayanfar A, Jouyban A. Physicochemical characterization of a new cocrystal of ketoconazole. Powder Technol. 2014;262:242–8. https://doi.org/10.1016/j.powtec.2014.04.072.
Ganesh M, Ubaidulla U, Rathnam G, Jang HT. Chitosan-telmisartan polymeric cocrystals for improving oral absorption: In vitro and in vivo evaluation. Int J Biol Macromol. 2019;131:879–85. https://doi.org/10.1016/j.ijbiomac.2019.03.141.
Mutalik S, Anju P, Manoj K, Usha AN. Enhancement of dissolution rate and bioavailability of aceclofenac: a chitosan-based solvent change approach. Int J Pharm. 2008;350:279–90. https://doi.org/10.1016/j.ijpharm.2007.09.006.
Alvani A, Jouyban A, Shayanfar A. The effect of surfactant and polymer on solution stability and solubility of tadalafil-methylparaben cocrystal. J Mol Liq. 2019;281:86–92. https://doi.org/10.1016/j.molliq.2019.02.080.
Rahman Z, Agarabi C, Zidan AS, Khan SR, Khan MA. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. AAPS Pharm Sci Tech. 2011;12:693–704. https://doi.org/10.1208/s12249-011-9603-4.
Lyn LY, Sze HW, Rajendran A, Adinarayana G, Dua K, Garg S. Crystal modifications and dissolution rate of piroxicam. Acta Pharm. 2011;61:391–402. https://doi.org/10.2478/v10007-011-0037-z.
Zhang YX, Wang LY, Dai JK, Liu F, Li YT, Wu ZY, et al. The comparative study of cocrystal/salt in simultaneously improving solubility and permeability of acetazolamide. J Mol Str. 2019;1184:225–32. https://doi.org/10.1016/j.molstruc.2019.01.090.
Gautam MK, Besan M, Pandit D, Mandal S, Chadha R. Cocrystal of 5-fluorouracil: characterization and evaluation of biopharmaceutical parameters. AAPS Pharm Sci Tech. 2019;20:1–7. https://doi.org/10.1208/s12249-019-1360-9.
Kaleemullah M, Jiyauddin K, Thiban E, Rasha S, Al-Dhalli S, Budiasih S, et al. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharm J. 2017;25:770–9. https://doi.org/10.1016/j.jsps.2016.10.006.
Rençber S, Karavana SY, Özyazici M. Bioavailability file: ketoprofen. FABAD J Pharm Sci. 2009;34:203.
Bhatia M, Devi S. Development, characterisation and evaluation of PVP K-30/PEG solid dispersion containing ketoprofen. ACTA Pharm Sci. 2020;58. https://doi.org/10.23893/1307-2080.APS.05806.
Bhatia M, Devi R. Enhanced solubility and drug release of ketoprofen using lyophilized bovine serum albumin solid dispersion. ACTA Pharm Sci. 2019;57. https://doi.org/10.23893/1307-2080.APS.05703.
Hezave AZ, Aftab S, Esmaeilzadeh F. Micronization of ketoprofen by the rapid expansion of supercritical solution process. J Aerosol Sci. 2010;41:821–33.
Vittal GV, Deveswaran R, Bharath S, Basavaraj BV, Madhavan V. Formulation and characterization of ketoprofen liquisolid compacts by Box-Behnken design. Int J Pharm Investig. 2012;2:150. https://doi.org/10.4103/2230-973X.104398.
Nikumbh KV, Sevankar SG, Patil MP. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv. 2015;22:509–15. https://doi.org/10.3109/10717544.2013.859186.
Ambala R, Vemula SK. Formulation and characterization of ketoprofen emulgels. J Appl Pharm Sci. 2015;5:112–7. https://doi.org/10.7324/JAPS.2015.50717.
Attia MF, Anton N, Khan IU, Serra CA, Messaddeq N, Jakhmola A, et al. One-step synthesis of iron oxide polypyrrole nanoparticles encapsulating ketoprofen as model of hydrophobic drug. Int J Pharm. 2016;508:61–70. https://doi.org/10.1016/j.ijpharm.2016.04.073.
Gul R, Ahmed N, Ullah N, Khan MI, Elaissari A. Biodegradable ingredient-based emulgel loaded with ketoprofen nanoparticles. AAPS Pharm Sci Tech.2018;19:1869–81. https://doi.org/10.1208/s12249-018-0997-0.
Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. NANOMED- Nanotechnol. 2010;6:753–9. https://doi.org/10.1016/j.nano.2010.06.003.
Xi MM, Wang XY, Fang KQ, Gu Y. Study on the characteristics of pectin–ketoprofen for colon targeting in rats. Int J Pharm. 2005;298:91–7. https://doi.org/10.1016/j.ijpharm.2005.04.012.
Kluge J, Fusaro F, Casas N, Mazzotti M, Muhrer G. Production of PLGA micro-and nanocomposites by supercritical fluid extraction of emulsions: I. Encapsulation of lysozyme. J Supercrit Fluids. 2009;50:327–35. https://doi.org/10.1016/j.supflu.2009.05.002.
Perpétuo GL, Chierice GO, Ferreira LT, Fraga-Silva TF, Venturini J, Arruda MS, et al. A combined approach using differential scanning calorimetry with polarized light thermomicroscopy in the investigation of ketoprofen and nicotinamide cocrystal. Thermochim Acta. 2017;651:1–0. https://doi.org/10.1016/j.tca.2017.02.014.
Maheshwari C, André V, Reddy S, Roy L, Duarte T, Rodríguez-Hornedo N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: effect of coformer ionization, pH max and solute–solvent interactions. Cryst Eng Comm. 2012;14:4801–11. https://doi.org/10.1039/C2CE06615G.
Sathisaran I, Dalvi SV. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics. 2018;10:108. https://doi.org/10.3390/pharmaceutics10030108.
Sanphui P, Kumar SS, Nangia A. Pharmaceutical cocrystals of niclosamide. Cryst Growth Des. 2012;12:4588–99. https://doi.org/10.1021/cg300784v.
Bhogala BR, Basavoju S, Nangia A. Tape and layer structures in cocrystals of some di-and tricarboxylic acids with 44’-bipyridines isonicotinamide Bin ternary cocrystals. Cryst Eng Comm. 2005;7:551–62. https://doi.org/10.1039/B509162D.
Sun S, Zhang X, Cui J, Liang S. Identification of the Miller indices of a crystallographic plane: a tutorial and a comprehensive review on fundamental theory, universal methods based on different case studies and matters needing attention. Nanoscale. 2020;12:16657–77. https://doi.org/10.1039/D0NR03637D.
Yadav AV, Shete AS, Dabke AP, Kulkarni PV, Sakhare SS. Co-crystals: a novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J Pharm Sci. 2009;71:359. https://doi.org/10.4103/0250-474X.57283.
Stewart JJ. Stewart Computational Chemistry, MOPAC2016. Colorado Springs. 2016; http://OpenMOPAC.net.
Granovsky AA, Firefly version 8, http://classic.chem.msu.su/gran/firefly/index.html.
Lu T, Chen F. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33:580–92. https://doi.org/10.1002/jcc.22885.
Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–8. https://doi.org/10.1016/0263-7855(96)00018-5.
Sarkar A, Rohani S. Cocrystals of acyclovir with promising physicochemical properties. J Pharm Sci. 2015;104:98–105. https://doi.org/10.1002/jps.24248.
Farrag Y, Ide W, Montero B, Rico M, Rodríguez-Llamazares S, Barral L, et al. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int J Biol Macromol. 2018;114:426–33. https://doi.org/10.1016/j.ijbiomac.2018.03.134.
Luo Y, Chen S, Zhou J, Chen J, Tian L, Gao W, et al. Luteolin cocrystals: characterization, evaluation of solubility, oral bioavailability and theoretical calculation. J Drug Deliv Sci Technol. 2019;50:248–54. https://doi.org/10.1016/j.jddst.2019.02.004.
Chavan RR, Hosamani KM. Microwave-assisted synthesis, computational studies and antibacterial/anti-inflammatory activities of compounds based on coumarin-pyrazole hybrid. R Soc Open Sci. 2018;5:172435. https://doi.org/10.1098/rsos.172435.
Alshaikh RA, Essa EA, El Maghraby GM. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: caffeine as a melting point modulator. Int J Pharm. 2019;563:395–405. https://doi.org/10.1016/j.ijpharm.2019.04.024.
Shandil A, Yadav M, Sharma N, Nagpal K, Jindal DK, Deep A, et al. Targeting keratinocyte hyperproliferation, inflammation, oxidative species and microbial infection by biological macromolecule-based chitosan nanoparticle-mediated gallic acid–rutin combination for the treatment of psoriasis. Polym Bull. 2020;77:4713–38. https://doi.org/10.1007/s00289-019-02984-9.
Komakech R, Kim YG, Matsabisa GM, Kang Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn.(Fabaceae): a narrative review. Integr Med Res. 2019;8:181–6. https://doi.org/10.1016/j.imr.2019.07.002.
Mondal A, Maity TK, Bishayee A. Analgesic and anti-inflammatory activities of quercetin-3-methoxy-4′-glucosyl-7-glucoside isolated from Indian medicinal plant Melothria heterophylla. Medicines. 2019;6:59. https://doi.org/10.3390/medicines6020059.
Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharma J. 2012;20:63–7. https://doi.org/10.1016/j.jsps.2011.08.001.
Olbert M, Gdula-Argasińska J, Nowak G, Librowski T. Beneficial effect of nanoparticles over standard form of zinc oxide in enhancing the anti-inflammatory activity of ketoprofen in rats. Pharmacol Rep. 2017;69:679–82.
Kulkarni SK. Heat and other physiological stress-induced analgesia: catecholamine mediated and naloxone reversible response. Life Sci. 1980;27:185–8. https://doi.org/10.1016/0024-3205(80)90136-8.
Acknowledgements
The authors are highly grateful to Department of Pharmaceutical Sciences, Central Instrumentation Laboratory and Material Science Laboratory, Guru Jambheshwar University of Science and Technology, Hisar for providing necessary facilities to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The procedure followed for animal handling is in accordance with the protocol as approved for animal study (CPCSEA Reg. no-IAEC/2020/10-18) by the Institutional Animal Ethical Committee, Guru Jambheshwar University of Science and Technology, Hisar, India.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhatia, M., Kumar, A., Verma, V. et al. Development of ketoprofen-p-aminobenzoic acid co-crystal: formulation, characterization, optimization, and evaluation. Med Chem Res 30, 2090–2102 (2021). https://doi.org/10.1007/s00044-021-02794-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02794-7