Abstract
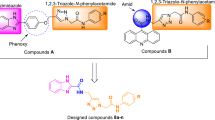
Benzoxazolyl linked meta- and para-substituted new chemical entities (5a–5h) featuring thiazolidinedione, rhodanine, hydantoin, and thiohydantoin moieties were synthesized and characterized by 1H NMR, 13C NMR, FT-IR, and HRMS spectral studies. In addition, all compounds were screened for α-glucosidase inhibitory activity and further supported by molecular docking studies carried out at the active site of α-glucosidase (PDB code: 3TOP) in comparison to acarbose used as a standard drug. Out of eight tested compounds, 5d was found as the most active inhibitor of α-glucosidase (IC50 = 9.48 ± 0.36 µM), having rhodanine moiety substituted at meta-position of the phenyl ring.




Similar content being viewed by others
References
Cho NH, Shaw JE, Karuranga S, Haung YH, RochaFernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. https://doi.org/10.1046/j.1464-5491.2000.00259.x.
Nguyen DV, Shawand LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol. 2012;3:170. doi: 10.3389%2Ffendo.2012.00170.
Zachary T, Bloomgarden MD. Approaches to treatment of type 2 diabetes. Diabetes Care. 2008;8:1697–703. https://doi.org/10.2337/dc08-zb08.
Chakrabarti R, Rajagopalan R. Diabetes and insulin resistance associated disorders: disease and the therapy. Curr Sci. 2002;83:1533–8. https://www.jstor.org/stable/24108177.
Martin AE, Montogomery PA. Acarbose: an alpha-glucosidase inhibitor. Am J Health Syst Pharm. 1996;53:2277–90. https://doi.org/10.1093/ajhp/53.19.2277.
Khan MS, Munawar MA, Ashraf M, Alam U, Ata A, Asiri AM, et al. Synthesis of novel indenoquinoxaline derivatives as potent α-glucosidase inhibitors. Bioorg Med Chem. 2014;22:1195–200. https://doi.org/10.1016/j.bmc.2013.12.024.
Pili R, Chang J, Partis RA, Mueller RA, Chrest FJ, Passaniti A. The alpha-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res. 1995;55:2920–6. https://pubmed.ncbi.nlm.nih.gov/7540952/.
Mehta A, Zitzmann N, Rudd PM, Block TM, Dwek RA. Alpha-glucosidase inhibitors as potential broad-based anti-viral agents. FEBS Lett. 1998;430:17–22. https://doi.org/10.1016/s0014-5793(98)00525-0.
Zitzmann N, Mehta AS, Carrouee S, Butters TM, Platt FM, McCauley J, et al. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc Natl Acad Sci USA. 1999;96:11878–82. https://doi.org/10.1073/pnas.96.21.11878.
Chinthala Y, Domatti AK, Sarfaraz A, Singh SP, Arigari NK, Gupta N, et al. Synthesis, biological evaluation and molecular modeling studies of some novel thiazolidinediones with triazole ring. Eur J Med Chem. 2013;70:308–14. https://doi.org/10.1016/j.ejmech.2013.10.005.
Wang G, Peng Y, Xie Z, Wang J, Chen M. Synthesis, α-glucosidase inhibition and molecular docking studies of novel thiazolidine-2,4-dione or rhodanine derivatives. Med Chem Commun. 2017;8:1477–84. https://doi.org/10.1039/C7MD00173H.
Wang G, Peng Z, Wang J, Li J, Li X. Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential α-glucosidase inhibitors. Bioorg Med Chem. 2016;24:5374–9. https://doi.org/10.1016/j.bmc.2016.08.061.
Singh G, Singh A, Verma RK, Mall R, Azeem U. Synthesis, biological evaluation and molecular docking studies of novel benzimidazole derivatives. Comp Biol Chem. 2018;72:45–52. https://doi.org/10.1016/j.compbiolchem.2017.12.010.
Singh V, Singh A, Singh G, Verma RK, Mall R. Novel benzoxazole derivatives featuring rhodanine and analogs as antihyperglycemic agents: synthesis, molecular docking, and biological studies. Med Chem Res. 2018;27:735–43. https://doi.org/10.1007/s00044-017-2097-1.
Kaur J, Singh A, Singh G, Verma RK, Mall R. Novel indolyl linked para-substituted benzylidene-based phenyl containing thiazolidinediones and their analogs as α-glucosidase inhibitors: synthesis, in vitro and molecular docking studies. Med Chem Res. 2018;27:903–14. https://doi.org/10.1007/s00044-017-2112-6.
Martínez-López D, Yu ML, García-Iriepa C, Campos PJ, Frutos ML, Golen JA, et al. Hydantoin-based molecular photoswitches. J Org chem. 2015;80:3929–39. https://doi.org/10.1021/acs.joc.5b00244.
Toubal K, Djafri A, Chouaih A, Talbi A. Synthesis and structural determination of novel 5-arylidene-3-N(2-alkyloxyaryl)-2-thioxothiazolidin-4-ones. Molecules. 2012;17:3501–9. https://doi.org/10.3390/molecules17033501.
Ishida T, In Y, Inoue M, Ueno Y, Tanaka C, Hamanaka N. Structural elucidation of epalrestat (ONO-2235), a potent aldose reductase inhibitor, and isomerization of its double bonds. Tetrahedron Lett. 1989;30:959–62. https://doi.org/10.1016/S0040-4039(00)95290-0.
Lazer ES, Miao CK, Wong HC, Sorcek R, Spero DM, Gilman A, et al. Benzoxazolamines and benzothiazolamines: potent, enantioselective inhibitors of leukotriene biosynthesis with a novel mechanism of action. J Med Chem. 1994;37:913–23. https://doi.org/10.1021/jm00033a008.
Mall R, Singh A, Singh G, Singh V, Verma RK. Indolyl linked meta‐substituted benzylidenes as novel ligands: synthesis, biological evaluation, and molecular docking studies. J Heterocycl Chem. 2019;56:1542–52. https://doi.org/10.1002/jhet.3529.
Granfeldt Y, Bjorck I, Drews A, Tovar J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur J Clin Nutr. 1992;46:649–60. https://pubmed.ncbi.nlm.nih.gov/1396482/.
Acknowledgements
The authors are highly thankful to Punjabi University Patiala authorities for providing the necessary research facilities. The authors also submit their sincere thanks to the Director and Mr. Avtar Singh of Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, for extending facilities for spectral analysis. Furthermore, VS acknowledges the University Grants Commission (UGC) for providing the Maulana Azad National Fellowship (Award number: MANF-2015-17-PUN-60098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Singh, V., Singh, A., Singh, G. et al. Benzoxazolyl linked benzylidene based rhodanine and analogs as novel antidiabetic agents: synthesis, molecular docking, and in vitro studies. Med Chem Res 30, 1905–1914 (2021). https://doi.org/10.1007/s00044-021-02781-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02781-y