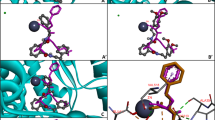
Abstract
Left ventricular hypertrophy (LVH) is a major adaptative response to the increase in the overload produced by hypertension, is a risk factor for myocardial infarction, stroke, and heart failure. Among the several factors involved in hypertension and in the progression to cardiac hypertrophy, the hyperactivity of the renin–angiotensin system (RAS) and the dysfunction of the neurovisceral/autonomic nervous system are the main mechanisms involved. Evidence demonstrates that the inhibition of RAS and the increase in parasympathetic activity reduce significantly LVH ameliorating cardiac function. The development of multi-target compounds is a relevant strategy for treating hypertension and cardiac hypertrophy. This study aimed to synthesize three phthalamide derivatives (M-01, M-02, and M-03) and evaluate them as three-target (ACE/AChE/BuChE) inhibitors with the possible dual effect of reducing hypertension and reverting cardiac hypertrophy. After in silico and in vitro experiments, one compound was tested in vivo on rats. All three phthalamides were synthesized in good yields, showing good competitive inhibition of the three-target enzymes in silico and in vitro. M-01 (10 mg/kg) significantly reversed cardiomyocite hypertrophy (by 87.3%; p < 0.001) in the heart of spontaneous hypertensive rat (SHR) model. It was at least 18-fold more potent than the reference drug (captopril), which provided only 32.7% reversion. Three-target inhibitory activity was herein demonstrated for M-01, M-02, and M-03 in vitro and in silico, each with a similar effect. The compound tested in vivo (M-01) exhibited great potency in reducing hypertension and reverting cardiomyocyte hypertrophy, making it a promising candidate for further research.

Highlights
-
The phthalamide derivatives are three-target inhibitors, acting on ACE, AChE, and BuChE.
-
The test compounds showed potency in treating hypertension and reverting cardiomyocyte hypertrophy.
-
M-01 was 18-fold more potent than captopril in reversing cardiomyocyte hypertrophy.







Similar content being viewed by others
References
Nawaz KAAA, David SM, Murugesh E, Thandeeswaran M, Kiran KG, Mahendran R, et al. Identification and in silico characterization of a novel peptide inhibitor of angiotensin converting enzyme from pigeon pea (Cajanus cajan). Phytomedicine. 2017;36:1–7. https://doi.org/10.1016/j.phymed.2017.09.013.
Agunloye OM, Oboh G, Ademiluyi AO, Ademosun AO, Akindahunsi AA, Oyagbemi AA, et al. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed Pharmacother. 2019;109:450–8. https://doi.org/10.1016/j.biopha.2018.10.044.
Magvanjav O, Cooper-DeHoff RM, McDonough CW, Gong Y, Segal MS, Hogan WR, et al. Antihypertensive therapy prescribing patterns and correlates of blood pressure control among hypertensive patients with chronic kidney disease. J Clin Hypertens. 2018. https://doi.org/10.1111/jch.13429.
Touyz RM. Hypertension guidelines: effect of blood pressure targets. Can J Cardiol. 2019;35:564–9. https://doi.org/10.1016/j.cjca.2019.03.014.
Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet.2016;388:2665–712. https://doi.org/10.1016/S0140-6736(16)31134-5.
Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens. 2009;27:963–75. https://doi.org/10.1097/HJH.0b013e3283282f65.
Campbell NRC, Niebylski ML. Prevention and control of hypertension. Curr Opin Cardiol. 2014;29:324–30. https://doi.org/10.1097/HCO.0000000000000067.
Te Riet L, Van Esch JHM, Roks AJM, Van Den Meiracker AH, Danser AHHJ. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–75. https://doi.org/10.1161/CIRCRESAHA.116.303587.
Mann SJ. Neurogenic hypertension: pathophysiology, diagnosis and management. Clin Auton Res. 2018;28:363–74. https://doi.org/10.1007/s10286-018-0541-z.
Takimoto-Ohnishi E, Murakami K. Renin–angiotensin system research: from molecules to the whole body. J Physiol Sci. 2019;69:581–7. https://doi.org/10.1007/s12576-019-00679-4.
Pellegrino PR, Schiller AM, Haack KKV, Zucker IH. Central angiotensin-II increases blood pressure and sympathetic outflow via Rho kinase activation in conscious rabbits. Hypertension. 2016;68:1271–80. https://doi.org/10.1161/HYPERTENSIONAHA.116.07792.
Alshak MN, M Das J. Neuroanatomy, Sympathetic Nervous System. StatPearls Publishing; 2019. Accessed 29 Oct 2020. http://www.ncbi.nlm.nih.gov/pubmed/31194352.
Benarroch EE. Physiology and pathophysiology of the autonomic nervous system. Contin Lifelong Learn Neurol. 2020;26:12–24. https://doi.org/10.1212/CON.0000000000000817.
Nwabuo CC, Vasan RS. Pathophysiology of hypertensive heart disease: beyond left ventricular hypertrophy. Curr Hypertens Rep. 2020;22. https://doi.org/10.1007/s11906-020-1017-9.
Lv Y, Li Y, Yi Y, Zhang L, Shi Q, Yang J, et al. A genomic survey of angiotensin-converting enzymes provides novel insights into their molecular evolution in vertebrates. Molecules.2018;23:2923. https://doi.org/10.3390/molecules23112923.
Panjaitan F, Gomez H, Chang Y-W, Panjaitan FCA, Gomez HLR, Chang Y-W. In silico analysis of bioactive peptides released from giant grouper (epinephelus lanceolatus) roe proteins identified by proteomics approach. Molecules. 2018;23:2910. https://doi.org/10.3390/molecules23112910.
Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg. 2014;3:2–7. https://doi.org/10.1016/j.amsu.2013.11.002.
Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22. https://doi.org/10.1016/0735-1097(93)90466-E.
Benedito S, Prieto D, Nielsen PJ, Berg Nyborg NC. Role of the endothelium in acetylcholine-induced relaxation and spontaneous tone of bovine isolated retinal small arteries. Exp Eye Res. 1991;52:575–9. https://doi.org/10.1016/0014-4835(91)90059-N.
Paton JFR, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain Res Rev. 2005;49:555–65. https://doi.org/10.1016/j.brainresrev.2005.02.005.
He B, Lu Z, He W, Huang B, Jiang H. Autonomic modulation by electrical stimulation of the parasympathetic nervous system: an emerging intervention for cardiovascular diseases. Cardiovasc Ther. 2016;34:167–71. https://doi.org/10.1111/1755-5922.12179.
Wilson BW. Cholinesterases. In: Krieger RI, Krieger WC, eds. Handbook of Pesticide Toxicology. Second. California: Academic Press, Elsevier; 2001:967–985. https://doi.org/10.1016/B978-012426260-7.50051-3.
Lataro RM, Silva CAA, Tefé-Silva C, Prado CM, Salgado HC. Acetylcholinesterase inhibition attenuates the development of hypertension and inflammation in spontaneously hypertensive rats. Am J Hypertens. 2015;28:1201–8. https://doi.org/10.1093/ajh/hpv017.
Milano GE, Leite N, Chaves TJ, Milano GE, Souza RLR, de, Alle LF. Butyrylcholinesterase activity and cardiovascular risk factors in obese adolescents submitted to an exercise program. Arq Bras Endocrinol Metabol. 2013;57:533–7. https://doi.org/10.1590/s0004-27302013000700006.
Blanco JHD, Gastaldi AC, Gardim CB, Araujo JE, Simões MV, Oliveira LFL, et al. Chronic cholinergic stimulation promotes changes in cardiovascular autonomic control in spontaneously hypertensive rats. Auton Neurosci Basic Clin. 2015;193:97–103. https://doi.org/10.1016/j.autneu.2015.09.002.
Yu F, Zhang Z, Luo L, Zhu J, Huang F, Yang Z, et al. Identification and molecular docking study of a novel angiotensin-i converting enzyme inhibitory peptide derived from enzymatic hydrolysates of cyclina sinensis. Mar Drugs. 2018;16:411. https://doi.org/10.3390/md16110411.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. (2009) Gaussian 09, Revision E. 01; Gaussian Inc., Wallingford CT (software).
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–62. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:143.0.CO;2-B.
Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc. 2016;11:905–19. https://doi.org/10.1038/nprot.2016.051.
Morris GM, Ruth H, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. https://doi.org/10.1002/jcc.21256.
Andrade-Jorge E, Rodríguez JE, Bribiesca-Carlos J, Gallardo-Ortíz IA, Trujillo-Ferrara JG, Villalobos-Molina R. Novel phthalamide derivatives as antihypertensive agents: rapid and clean synthesis, in silico and in vivo evaluation. Med Chem Res. 2019;28:681–95. https://doi.org/10.1007/s00044-019-02327-3.
Ruiz-Maciel O, Padilla-Martínez II, Sánchez-Labastida LA, Soriano-Ursúa MA, Andrade-Jorge E, Trujillo-Ferrara JG. Inhibitory activity on cholinesterases produced by aryl-phthalimide derivatives: green synthesis, in silico and in vitro evaluation. Med Chem Res. 2020;29:1030–40. https://doi.org/10.1007/s00044-020-02543-2.
Andrade-Jorge E, Bribiesca-Carlos J, Martínez-Martínez FJ, Soriano-Ursúa MA. Padilla-Martínez II, Trujillo-Ferrara JG. Crystal structure, DFT calculations and evaluation of 2-(2-(3,4-dimethoxyphenyl)ethyl)isoindoline-1,3-dione as AChE inhibitor. Chem Cent J. 2018;12:74. https://doi.org/10.1186/s13065-018-0442-1.
Bonting SL, Featherstone RM. Ultramicro assay of the cholinesterases. Arch Biochem Biophys. 1956;61:89–98. https://doi.org/10.1016/0003-9861(56)90319-8.
Andrade-Jorge E, Sánchez-Labastida LA, Soriano-Ursúa MA, Guevara-Salazar JA, Trujillo-Ferrara JG. Isoindolines/isoindoline-1,3-diones as AChE inhibitors against Alzheimer’s disease, evaluated by an improved ultra-micro assay. Med Chem Res. 2018;27:2187–98. https://doi.org/10.1007/s00044-018-2226-5.
Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010:475–90. https://doi.org/10.7150/ijbs.6.475.
Henda Y Ben, Labidi A, Arnaudin I, Bridiau N, Delatouche R, Maugard T, et al. Measuring angiotensin-I converting enzyme inhibitory activity by micro plate assays: Comparison using marine cryptides and tentative threshold determinations with captopril and losartan. J Agric Food Chem. 2013;61:10685–90. https://doi.org/10.1021/jf403004e.
Sui X, Gao C. Huperzine A ameliorates damage induced by acute myocardial infarction in rats through antioxidant, anti-apoptotic and anti-inflammatory mechanisms. Int J Mol Med. 2014;33:227–33. https://doi.org/10.3892/ijmm.2013.1546.
Rocha JA, Ribeiro SP, França CM, Coelho O, Alves G, Lacchini S, et al. Increase in cholinergic modulation with pyridostigmine induces anti-inflammatory cell recruitment soon after acute myocardial infarction in rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R697–706. https://doi.org/10.1152/ajpregu.00328.2015.
Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol. 2018;10:17–28.
Rocha WA, Lunz W, Baldo MP, Pimentel EB, Dantas EM, Rodrigues SL, et al. Kinetics of cardiac and vascular remodeling by spontaneously hypertensive rats after discontinuation of long-term captopril treatment. Braz J Med Biol Res. 2010;43:390–6. https://doi.org/10.1590/S0100-879X2010007500023.
Matsushima Y, Kawamura M, Akabane S, Imanishi M, Kuramochi M, Ito K, et al. Increases in renal angiotensin II content and tubular angiotensin II receptors in prehypertensive spontaneously hypertensive rats. J Hypertens. 1988;6:791–6. https://doi.org/10.1097/00004872-198810000-00005.
Haddad G, Garcia R. Effect of angiotensin-converting enzyme two-week inhibition on renal angiotensin II receptors and renal vascular reactivity in SHR. J Mol Cell Cardiol. 1997;29:813–22. https://doi.org/10.1006/jmcc.1996.0304.
Shanks J, Manou-Stathopoulou S, Lu C-J, Li D, Paterson DJ, Herring N. Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am J Physiol Circ Physiol. 2013;305:H980–6. https://doi.org/10.1152/ajpheart.00255.2013.
Katayama S, Abe M, Inaba M, Itabashi A, Ishii J. Effects of captopril or nitrendipine on left ventricular collagen or laminin B2 gene expression. Jpn Heart J. 1993;34:773–83. https://doi.org/10.1536/ihj.34.773.
Rodríguez JE, Saucedo-Campos AD, Vega AV, et al. The α1D-adrenoreceptor antagonist BMY 7378 reverses cardiac hypertrophy in spontaneously hypertensive rats. J Hypertens. 2020:1. https://doi.org/10.1097/HJH.0000000000002412.
Rocha-Resende C, Roy A, Resende R, Ladeira MS, Lara A, de Morais Gomes ER, et al. Non-neuronal cholinergic machinery present in cardiomyocytes offsets hypertrophic signals. J Mol Cell Cardiol. 2012;53:206–16. https://doi.org/10.1016/j.yjmcc.2012.05.003.
Acknowledgements
EAJ received a postdoctoral fellowship from DGAPA of the Universidad Nacional Autónoma de México (UNAM). Additionally, this work was supported by CONACYT-México, SIP-projects (M1930, and 20201031) from the Instituto Politécnico Nacional-ESM, and RVM and IAGO received grants from PAPIIT (IN223519 and IN226819, respectively). We thank MVZ L. Flores, MD. F. Barrón-Moreno, C.Y. Gallegos-Quiroz, and Biol. TE Villamar-Duque from Facultad de Estudios Superiores Iztacala (FESI), UNAM, for their aid in animal care and housing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Andrade-Jorge, E., Rodríguez, J.E., Lagos-Cruz, J.A. et al. Phthalamide derivatives as ACE/AChE/BuChE inhibitors against cardiac hypertrophy: an in silico, in vitro, and in vivo modeling approach. Med Chem Res 30, 964–976 (2021). https://doi.org/10.1007/s00044-021-02707-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02707-8