Abstract
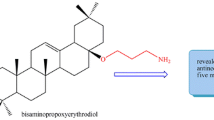
The first representatives of F16-conjugated pentacyclic triterpenoids, betulin and betulinic, ursolic, oleanolic, and glycyrrhetic acid derivatives, were synthesized. The triterpene core was linked, at the С-3, С-28, or С-30 position, to one or two mitochondria-targeting delocalized lipophilic cations F16 via butane or triethylene glycol spacer. The human cancer cell lines U937 (leukemic monocyte lymphoma), K562 (chronic myeloid leukemia), and Jurkat (T-lymphoblastic leukemia), and a human nonmalignant fibroblast cell line were used to evaluate the cytotoxic activities of the products. Most of the obtained conjugates showed considerable enhancement of the antitumor action in comparison with the parent betulinic acid (≈100−200-fold) and a markedly higher cytotoxic effect against tumor cell lines over healthy fibroblast cells. In the series of test compounds, F16 conjugates with betulin and betulinic acid 6, 8, and 11 were most selective, showing acceptable values of selectivity index (≥10).







Similar content being viewed by others
References
Smith RA, Hartley RC, Cochemé HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012;33:341–52.
Tatarkova Z, Kuka S, Petráš M, Račay P, Lehotský J, Dobrota D, et al. Why mitochondria are excellent targets for cancer therapy. Klin Onkol. 2012;25:421–6.
Gogvadze V. Targeting mitochondria in fighting cancer. Curr Pharm Des. 2011;17:4034–46.
Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–73.
Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70.
Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria. Adv Drug Deliv Rev. 2009;61:1250–75.
Wang F, Ogasawara MA, Huang P. Small mitochondria-targeting molecules as anti-cancer agents. Mol Aspects Med. 2010;31:75–92.
Reddy CA, Somepalli V, Golakoti T, Kanugula AK, Karnewar S, Rajendiran K, et al. Mitochondrial-targeted curcuminoids: a strategy to enhance bioavailability and anticancer efficacy of curcumin. PLOS ONE. 2014;9:e89351.
Strobykina IY, Belenok MG, Semenova MN, Semenov VV, Babaev VM, Rizvanov IK, et al. Triphenylphosphonium cations of the diterpenoid isosteviol: synthesis and antimitotic activity in a sea urchin embryo model. J Nat Prod. 2015;78:1300–8.
Tsepaeva OV, Nemtarev AV, Abdullin TI, Grigor’eva LR, Kuznetsova EV, Akhmadishina RA, et al. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J Nat Prod. 2017;80:2232–9.
Ye Y, Zhang T, Yuan H, Li D, Lou H, Fan P. Mitochondria-targeted lupane triterpenoid derivatives and their selective apoptosis-inducing anticancer mechanisms. J Med Chem. 2017;60:6353–6363.
Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, et al. Mitochondria targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017;117:10043–120.
Hussain H, Green IR, Ali I, Khan IA, Ali Z, Al-Sadi AM, et al. Ursolic acid derivatives for pharmaceutical use: a patent review (2012–2016). Expert Opin Ther Pat. 2017;27:1061–72.
Kessler JH, Mullauer FB, Roo GM, Medema JP. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007;251:132–45.
Lin C, Wen X, Sun H. Oleanolic acid derivatives for pharmaceutical use: a patent review. Expert Opin Ther Pat. 2016;26:643–55.
Mullauer FB, Kessler JH, Medema JP. Betulinic acid, a natural compound with potent anti-cancer effects. Anticancer Drugs. 2010;21:215–27.
Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Clin Cancer Res. 2007;5:943–55.
Csuk R. Betulinic acid and its derivatives: a patent review (2008–2013). Expert Opin Ther Pat. 2014;24:913–23.
Zhang X, Hu J, Chen Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol Med Rep. 2016;14:4489–95.
Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–90.
Fulda S, Kroemer G. Mitochondria as therapeutic targets for the treatment of malignant disease. Antioxid Redox Signal. 2011;15:2937–49.
Nedopekina DA, Gubaidullin RR, Odinokov VN, Maximchik PV, Zhivotovsky B, Bel’skii YP, et al. Mitochondria-targeted betulinic and ursolic acid derivatives: synthesis and anticancer activity. Med Chem Comm. 2017;8:1934–45.
Spivak AYU, Nedopekina DA, Khalitova RR, Gubaidullin RR, Odinokov VN, Bel’skii YP, et al. Triphenylphosphonium cations of betulinic acid derivatives: synthesis and antitumor activity. Med Chem Res. 2017;26:518–31.
Spivak AYU, Nedopekina DA, Shakurova ER, Khalitova RR, Gubaidullin RR, Odinokov VN, et al. Synthesis of lupane triterpenoids with triphenylphosphonium substituents and studies of their antitumor activity. Russ Chem Bull. 2013;62:188–98
Sommerwerk S, Heller L, Kerzig C, Kramell AE, Csuk R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur J Med Chem. 2017;127:1–9.
Fantin VR, Berardi MJ, Scorrano L, Korsmeyer SJ, Leder P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell. 2002;2:29–42.
Fantin VR, Leder P. F16, a mitochondriotoxic compound, triggers apoptosis or necrosis depending on the genetic background of the target carcinoma cell. Cancer Res. 2004;64:329–36.
Peng YB, Zhao ZL, Liu T, Xie GJ, Jin C, Deng TG, et al. A multi-mitochondrial anticancer agent that selectively kills cancer cells and overcomes drug resistance. Chem Med Chem. 2017;12:250–6.
Wang J, Fan X-Y, Yang L-YU, He H, Huang R, Jiang F-L, et al. Conjugated 5-fluorouracil with mitochondria-targeting lipophilic cation: design, synthesis and biological evaluation. Med Chem Commun. 2016;7:2016–9.
Kim DSHL, Chen Z, Nguyen T, Pezzuto JM, Qiu S, Lu Z-Z. A concise semi-synthetic approach to betulinic acid from botulin. Synth Commun. 1997;27:1607–12.
Xiang C, Li DW, Qi ZD, Jiang FL, Ge YS, Liu Y. Synthesis of F16 conjugated with 5-fluorouracil and biophysical investigation of its interaction with bovine serum albumin by a spectroscopic and molecular modeling approach. Luminescence. 2012;28:865–72.
Zhang X-H, Wang L-Y, Zhai G-H, Wen Z-Y, Zhang Z-X. Microwave-assisted solvent-free synthesis of some dimethine cyanine dyes, spectral properties and TD-DFT/PCM calculations. Bull Korean Chem Soc. 2007;28:2382–8.
Guo L, Chan MS, Xu D, Tam DY, Bolze F, Lo PK, et al. Indole-based cyanine as a nuclear RNA-selective two-photon fluorescent probe for live cell imaging. ACS Chem Biol. 2015;10:1171–5.
Suzuki K, Kobayashi A, Kaneko S, Takehira K, Yoshihara T, Ishida H, et al. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys Chem Chem Phys. 2009;11:9850–60.
Bernardo TC, Cunha-Oliveira T, Serafim TL, Holy J, Krasutsky D, Kolomitsyna O, et al. Dimethylaminopyridine derivatives of lupane triterpenoids cause mitochondrial disruption and induce the permeability transition. Bioorg Med Chem. 2013;21:7239–49.
Acknowledgements
This work was performed under financial support from the Russian Science Foundation (Grant 19-73-00155). The structural studies of the synthesized compounds were performed with the use of Collective Usage Centre “Agidel” at the Institute of Petrochemistry and Catalysis of RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Spivak, A.Y., Nedopekina, D.A., Gubaidullin, R.R. et al. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med Chem Res 30, 940–951 (2021). https://doi.org/10.1007/s00044-021-02702-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02702-z