Abstract
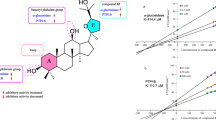
Dammarenolic acid (DA) is an A-seco-dammarane triterpenoids, isolated from Dipterocarpus alatus resin. DA was modified including reactions of esterification and amination with heterocyclic amines and l-amino acids. The structures of new compounds were confirmed by MS, 1H NMR, and 13C NMR spectroscopic analyses and their activities against α-glucosidase and acetylcholinesteras were studied. The cytotoxic activity of DA was screened using a broad panel of 60 human cancer cell lines and it has cytotoxic effect for a variety of human tumor cell lines (leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, breast cancer). All A-seco-dammarane derivatives exhibited very low or no activity against achetylcholinesterase. The majority of new compounds demonstrated higher antidiabetic activity against α-glucosidase compared with starting dammarenolic acid. The methyl dammarenoloate determined as a lead compound with the IC50 value of 0.037 μM being about 4800-fold more active than acarbose and 108-fold more active than the native DA with the IC50 value of 4.0 μM.


Similar content being viewed by others
References
Akihisa T, Ogihara J, Kato J, Yasukawa K, Ukiya M, Yamanouchi S, Oishi K (2001) Inhibitory effects of triterpenoids and sterols on human immunodeficiency virus-1 reverse transcriptase. Lipids 36:507–512
Akihisa T, Tokuda H, Ukiya M, Suzuki T, Enjo F, Koike K, Nikaido T, Nishino H (2004) 3-epicabraleahydroxy lactone and other triterpenoidsfrom Camellia oil and their inhibitory effects on Epstein–Barr virus activation. Chem Pharm Bull 52:153–156
Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatricmemory dysfunction. Science 217:408–414
Cao J, Zhang X, Qu F, Guo Z, Zhao Y (2015) Dammarane triterpenoids for pharmaceutical use: a patent review (2005–2014). Expert Opin Ther Pat 25:805–817
Chen L, Qiu W, Tang J, Wang ZF, He SY (2011) Synthesis and bioactivity of novel nitric oxide-releasing ursolic acid derivatives. Chin Chem Lett 22(4):413–416
Coy JF, Dressler D, Wilder J, Schubert P (2005) Mutation in the transketolase-like gene TKTL1: clinical implications for neurodegenerative disease, diabetes and cancer. Clin Lab 51:257–273
Do HTT, Tran TTT, Tran TH, Nguyen TT, Nguyen QT, Smirnova IE, Kazakova OB, Minnibaeva EM, Tolstikov GA (2013) Sinthesis and cytotoxity of derivatives of dipterocarpol, metabolite of Dipterocarpus alatus. Chem Nat Comp 49:58–65
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Bio Pharm 7:88–95
Elumalai K, Ali MA, Elumalai M, Kalpana Eluri K, Srinivasan S (2015) Acetylcholinesterase enzyme inhibitor activity of some novel pyrazinamide condensed 1,2,3,4-tetrahydropyrimidines. Biotechnol Rep 5:1–6
Esimone CO, Eck G, Duong TN, Uberla K, Proksch P, Grunwald T (2008) Potential anti-respiratory syncytial virus lead compounds from Aglaia species. Pharmazie 63:768–773
Esimone CO, Eck G, Nworu CS, Hoffmann D, Uberla K, Proksch P (2010) Dammarenolic acid, a seco dammarane triterpenoid from Aglaia sp. shows potentanti-retroviralactivity in vitro. Phytomedicine 17:540–547
Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor CO (1989) Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA 262:2551–2556
Grossberg GT (2003) Cholinesterase inhibitors for the treatment of Alzheimer’s disease. Curr Ther Res Clin Exp 64:216–235
Hu Y, Zhang J, Chandrashankra O, Fanny CFIp, Nancy YI (2013) Design, synthesis and evaluation of novel heterodimers of donepezil and huperzine fragments as acetylcholinesterase inhibitors. Bioorg Med Chem 21:676–683
Inada A, Somekawa M, Murata H, Nakanishi T, Tokuda H, Nishino H, Iwashima A, Darnaedi D, Murata J (1993) Phytochemical studies on meliaceous plants. VIII. Structures and inhibitory effects of Epstein–Barr virus activation of triterpenoids from leaves of Chisocheton macrophyllus King. Chem Pharm Bull 41:617–619
Kahnt M, Fischer L, Al-Harrasi A, Csuk R (2018) Ethylenediamine derived carboxamides of betulinic and ursolic acid as potential cytotoxic agents. Molecules 23:e2558
Kim YM, Wang MH, Rhee HI (2004) A novel α-glucosidase inhibitor from pine bark. Carbohydr Res 339:715–717
Khusnutdinova EF, Smirnova IE, Giniyatullina GV, Medvedeva NI, Yamansarov EY, Kazakov DV, Kazakova OB, Linh PT, Viet Q, Do TTH (2016) Inhibition of alpha-glucosidase by synthetic derivatives of lupane, oleanane, ursane and dammarane triterpenoids. Nat Prod Commun 11:33–35
Khusnutdinova EF, Smirnova IE, Kazakova OB, Petrova AV, Nguyen TTH, Do QV (2017) Synthesis and evaluation of 2,3-indolotriterpenoids as a new α-glucosidase inhibitor. Med Chem Res 26:2737–2742
Li T, Zhang XD, Song YW, Liu JW (2005) A microplate-based screening method for α-glucosidase inhibitors. Nat Prod Res Dev 10:1128–1134
Liby KT, Sporn MB (2012) Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharm Rev 64:A–AF
Mills JS, Werner AEA (1955) The chemistry of dammar resin. J Chem Soc 3132–3140. https://pubs.rsc.org/en/content/articlelanding/1955/JR/jr9550003132#!divAbstract
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766
Moura MB, Santos LS, Houten BV (2010) Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagenesis 51:391–405
Poehland BL, Carté BK, Francis TA, Hyland LJ, Allaudeen HS, Troupe N (1987) In vitro antiviral activity of dammar resin triterpenoids. J Nat Prod 50:706–713
Popov SA, Semenova MD, Baev DS, Sorokina IV, Zhukova NA, Frolova TS, Tolstikova TG, Shults EE, Māris Turks M (2019) Lupane-type conjugates with aminoacids, 1,3,4- oxadiazole and 1,2,5-oxadiazole-2-oxide derivatives: Synthesis, anti-inflammatory activity and in silico evaluation of target affinity. Steroids 150:108443–108453
Révész L, Hiestand P, La Vecchia L, Naef R, Naegeli HU, Oberer L, Roth HJ (1999) Isolation and synthesis of a novel immunosuppressive 17 alpha-substituted dammarane from the flour of the palmyrah palm (Borassus flabellifer). Bioorg Med Chem Lett 9:1521–1526
Saito H, Tsuchiya M, Naka S, Takagi K (1977) Effect of panax ginseng root on conditioned avoidance response in rats. Jpn J Pharm 27:509–516
Scholz D, Baumann K, Grassberger M, Wolff-Winiski B, Rihs G, Walter H, Meingassner JG (2004) Synthesis of dammarane-type triterpenoids with anti-inflammatory activity in vivo. Bioorg Med Chem Lett 14:2983–2986
Shanmugam MK, Nguyen AH, Alan P, Kumar AP, Tan BKH, Sethi G (2012) Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: potential role in prevention and therapy of cancer. Cancer Lett 320:158–170
Smirnova IE, Do Thi Thu H, Kazakova OB, Tolstikov GA, Kukovinetz OS, Lobov AN, Syponitskii KYu. (2012) Ozonolisys of dipterocarpol and its derivatives. Russ J Org Chem 48:1374–1379
Smirnova IE, Kazakova OB, Do TTH, Minnibaeva EM, Lobov AN, Suponitsky KYu. (2014) One-pot synthesis of hollongdione from dipterocarpol. Nat Prod Comm 9:1417–1420
Smirnova IE, Khusnutdinova EF, Lobov AN, Kazakova OB (2018) Synthesis of new A-conjugated quinolone and spiroindole dammaranes by the ozonolysis of 2,3-indolodipterocarpol. Nat Prod Comm 13:581–584
Sousa JLC, Freire CSR, Armando JD, Silvestre AJD, Silva AMS (2019) Recent developments in the functionalization of betulinic acid and its natural analogues: a route to new bioactive compounds. Molecules 24:355–379
Sun Y, Lai MS, Lu CJ, Chen RC (2008) How long can patients with mild or moderate Alzheimer’s dementia maintain both the cognition and the therapy of cholinesterase inhibitors: a national population-based study. Eur J Neurol 15:278–283
Tian Z, Si L, Skehan P, Meng K, Zhou X, Zhang Y, Zhou D, Xiao S (2017) Inhibition of influenza virus infection by multivalent pentacyclic triterpene-functionalized per-O-methylated cyclodextrin conjugated. Eur J Med Chem 134:133–139
Ukiya M, Kikuchi T, Tokuda H, Tabata K, Kimura Y, Arai T, Ezaki Y, Oseto O, Suzuki T, Akihisa T (2010) Antitumor-promoting effects and cytotoxic activities of dammar resin triterpenoids and their derivatives. Chem Biodivers 7:1871–1884
Zhou F, Wu GR, De-Cai DS, Xu B, Yan MM, Tao M, Guo WB, Zhang WX, Huang XM, Jia XH, Yang YQ, Gao F, Wang PL, Lei HM (2019) Synthesis and biological activity of glycyrrhetinic acid derivatives as antitumor agents. Eur J Med Chem 178:623–635
Acknowledgements
The work was carried out under the financial support of the Russian Foundation for Basic Research (project no. 18-53-54005) and the joint-project between Vietnam Academy of Science and Technology (VAST) and Russian Foundation for Basic Research (Project Nr. QTRY01.02/18-19). We thank the National Cancer Institute for the screening of compound 1 on human cancer cell lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Smirnova, I.E., Petrova, A.V., Kazakova, O.B. et al. Synthesis of dammarenolic acid derivatives with a potent α-glucosidase inhibitory activity. Med Chem Res 29, 94–102 (2020). https://doi.org/10.1007/s00044-019-02462-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02462-x