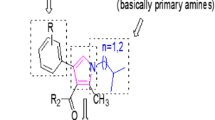
Abstract
We have synthesized nineteen (1–19) bisindolylmethane sulfonamide analogs, characterized by different spectroscopic techniques such as 1HNMR and EI-MS and tested for α-amylase inhibitory potential. All compounds showed excellent to moderate degree of α-amylase inhibitory potential with IC50 values ranging between 1.192 ± 0.51 to 3.057 ± 0.18 μM as equated with standard acarbose (IC50 values 0.83 ± 0.36 μM). Among the series, six analogs such as 1, 4, 5, 6, 10, and 14 showed potent α-amylase inhibition with IC50 values 1.747 ± 0.2, 1.208 ± 0.15, 1.192 ± 0.51, 1.858 ± 0.08, 1.358 ± 0.27 and 1.527 ± 0.17 μM, respectively, as equated with standard acarbose. The structure-activity relationship based upon different substituents on phenyl part. Molecular docking studies performed to recognize the binding interaction of the most active compounds.








Similar content being viewed by others

References
Adegboye AA, Khan KM, Salar U, Aboaba SA, Chigurupati S, Fatima I, Taha M, Wadood A, Mohammad JI, Khan H, Perveen S (2018) 2-Aryl benzimidazoles: synthesis, in vitro α-amylase inhibitory activity, and molecular docking study. Eur J Med Chem 150:248–260
Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C (2011) Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr 66:143–148
Ali S, Ali N, Ahmad DB, Pradhan V, Farooqui M (2013) Chemistry and biology of indoles and indazoles: a mini review. Med Chem 13:1792–1800
Anouar EH, Raweh S, Bayach I, Taha M, Baharudin MS, Meo FD, Hasan MH, Adam A, Ismail NH, Weber JF, Trouillas P (2013) Antioxidant properties of phenolic Schiff bases: structure-activity relationship and mechanism of action. J Comput Aided Mol Des 27:951–964
Asghari A, Ameri M, Radmannia S, Rajabi M, Bakherad M, Nematollahi D (2014) None-catalyst and clean synthesis of symmetric and asymmetric indoles from electrochemical oxidation of 4-aminophenol and p-phenylenediamine in the presence of malononitrile in green media. J Electro Chem 733:47–52
Aziz AN, Taha M, Ismail NH, Anouar EH, Yousuf S, Jamil W, Awang K, Ahmat N, Khan KM, Kashif SM (2014) Synthesis, crystal structure, DFT studies and evaluation of the antioxidant activity of 3,4-dimethoxybenzenamine schiff bases. Molecules 19:8414–8433
Bale AT, Khan KM, Salar U, Chigurupati S, Fasina T, Ali F, Wadood A, Taha M, Nanda SS, Ghufran M, Perveen S (2018) Chalcones and bis-chalcones: as potential α-amylase inhibitors; synthesis, in vitro screening, and molecular modelling studies. Bioorg Chem 79:179–189
Gollapalli M, Taha M, Ullah H, Nawaz M, AlMuqarrabun LMR, Rahim F, Qureshi F, Mosaddik A, Ahmat N, Khan KM (2018) Synthesis of bis-indolylmethane sulfonohydrazides derivatives as potent α-glucosidase inhibitors. Bioorg Chem 80:112–120
Imran S, Taha M, Selvaraj M, Ismail NH, Chigurupati S, Mohammad JI (2017) Synthesis and biological evaluation of indole derivatives as α-amylase inhibitor. Bioorg Chem 73:121–128
Jarald E, Joshi SB, Jain DC (2008) Diabetes and herbal medicines. Iran J Pharma Ther 7:97–106
Javid MT, Rahim F, Taha M, Nawaz M, Wadood A, Ali M, Mosaddik A, Shah SAA, Farooq RK (2018) Synthesis, SAR elucidations and molecular docking study of newly designed isatin based oxadiazole analogs as potent inhibitors of thymidine phosphorylase. Bioorg Chem 79:323–333
Khan KM, Taha M, Naz F, Khan M, Rahim F, Samreen, Perveen S, Choudhary MI (2011) Synthesis and in vitro leishmanicidal activity of disulfide derivatives. Med Chem 7:704–710
Khan KM, Taha M, Rahim F, Ali M, Jamil W, Perveen S, Choudhary MI (2010) An improved method for the synthesis of disulfides by periodic acid and sodium hydrogen sulfite in water. Lett Org Chem 7:244
Khan KM, Taha M, Ali M, Perveen S (2009) A mild and alternative approach towards symmetrical disulfides using H3IO5/NaHSO3 combination. Lett Org Chem 6:319–320
Khan KM, Ali M, Taha M, Perveen S, Choudhary MI, Voelter W (2008) An expedient and selective approach towards disulfides using sodium bromate/sodium hydrogen sulfite reagent. Lett Org Chem 5:432–434
Khan KM, Naz F, Taha M, Khan A, Perveen S, Choudhary MI, Voelter W (2014) Synthesis and in vitro urease inhibitory activity of N,N’-disubsituted thioureas. Eur J Med Chem 74:314–323
Kwon YI, Vattem DA, Shetty K (2006) Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pacif J Clin Nutr 15:107–118
Liu T, Song L, Wang H, Huang D (2011) A high-throughput assay for quantification of starch hydrolase inhibition based on turbidity measurement. J Agric Food Chem 59:9756–9762
Musharraf SG, Bibi A, Shahid N, Najam-ul-Haq M, Khan M, Taha M, Mughal UR, Khan KM (2012) Acylhydrazide and isatin schiff bases as alternate UV laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am J Ana Chem 3:779–789
Nencki M (1874) On a combination of sulphocarbamide with ethyl oxalate. Deut Chem Ges Ber vii:779–780
Noreen T, Taha M, Imran S, Chigurupati S, Rahim F, Selvaraj M, Ismail NH, Mohammad JI, Ullah H, Nawaz F, Irshad M, Ali M (2017) Synthesis of alpha amylase inhibitors based on privileged indole scaffold. Bioorg Chem 72:248–255
Nematollahi D, Hedayatfar V (2011) Diversity in electrochemical oxidation of dihydroxybenzenes in the presence of 1-methylindole. J Chem Sci 123:709–717
Pelletier SW (1999) Alkaloids: Chemical and Biological Perspectives. Springer-Verlag, New York, NY, Springer
Sales PM, de Souza PM, Simeoni LA, Magalhães PDO, Silveira D (2012) α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharmace Sci 15:141–183
Salar U, Khan KM, Chigurupati S, Taha M, Wadood A, Vijayabalan S, Ghufran M, Perveen S (2017) New hybrid hydrazinylthiazole substituted chromones: as potential α-amylase inhibitors and radical (DPPH & ABTS) scavengers. Sci Rep 7:16980
Shaheen RM, Davis DW, Liu W (1999) Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastases and induces tumor and endothelial cell apoptosis. Cancer Res 59:5412–5416
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) α-Amylases from microbial sources–An overview on recent developments. Food Techn Biotech 44:173–184
Stöckly F (1881) Zur Kenntniss der Fäulnissprodukte des Gehirns. J Prakt Chem 24:17–24
Sundberg RJ (1996) The Chemistry of Indoles. Academic Press, New York, NY
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitam 52:149–153
Taha M, Naz H, Rasheed S, Ismail NH, Rahman AA, Yousuf S, Choudhary MI (2014) Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules 19:1286–1301
Taha M, Ismail NH, Jamil W, Yousuf S, Jaafar FM, Ali MI, Kashif SM, Hussain E (2013) SynthEsis, Evaluation Of Antioxidant Activity And Crystal Structure Of 2,4-dimethylbenzoylhydrazones. Molecules 18:10912–10929
Taha M, Javid MT, Imran S, Selvaraj M, Chigurupati S, Ullah H, Rahim F, Khan F, Mohammad JI (2017) Synthesis and study of the α-amylase inhibitory potential of thiadiazole quinoline derivatives. Bioorg Chem 74:179–186
Taha M, Irshad M, Imran S, Rahim F, Selvaraj M, Almandil NB, Ibrahim M (2019) Thiazole based carbohydrazide derivatives as α-amylase inhibitor and their molecular docking study. Hetero Chem 2019:7502347
Zhang L, Hogan S, Li J, Sun S, Canning C, Zheng SJ, Zhou K (2011) Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mic. Food Chem 126:466–471
Acknowledgements
We would like to thank IRMC and Imam Abdulrahman Bin Faisal University for Lab facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Taha, M., Noreen, T., Imran, S. et al. Synthesis, α-amylase inhibition and molecular docking study of bisindolylmethane sulfonamide derivatives. Med Chem Res 28, 2010–2022 (2019). https://doi.org/10.1007/s00044-019-02431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02431-4