Abstract
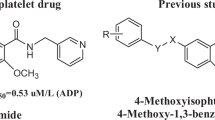
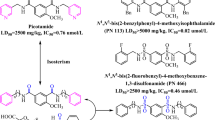
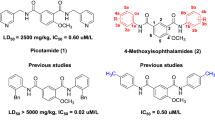
According to the bio-isosterism theory, a series of N, N’-disubstitutedphenyl-4-ethoxylbenzene-1, 3-disulfonamides (5a-p) were designed and synthesized by two steps of reactions including chlorosulfonation and ammonolysis. The structures of all compounds have been confirmed by IR, 1H-NMR, 13C-NMR, and ESI-MS spectra. The in vitro anti-platelet aggregation activities were evaluated by Born’s test induced by adenosine diphosphate (ADP) and arachidonic acid (AA), respectively. The biological evaluation results revealed that compound 5h had the lowest IC50 value (0.32 μM) and the highest inhibition rate (40.9 %) that of three positive control agents clopidogrel (0.41 μM, 23.5 %), aspirin (0.53 μM, 28.9 %), and picotamide (0.76 μM, 32.7 %). Afterwards, compounds with higher activities were selected to further study in vitro cytotoxicity via cell counting kit-8 (CCK-8) assay. The cytotoxicity results indicated that compound 5h had simultaneously the lowest cytotoxicity, while other compounds had no significant relationship between the anti-platelet activities and cytotoxicities. Based on above in vitro anti-platelet activity data, the SAR (Structure Activity Relationship) of the target compounds was preliminarily summarized. In general, N, N’-disubstitutedphenyl-4-ethoxylbenzene-1, 3-disulfonamides have the potential of further study and very likely become safer and more effective anti-platelet agents.






Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- AA:
-
Arachidonic acid
- SAR:
-
Structure activity relationship
- COX-I:
-
Cyclooxygenase-I
- TXA2 :
-
Thromboxane A2
- PGI2 :
-
Prostaglandin I2
- TLC:
-
Thin layer chromatography
- CCK-8:
-
Cell Counting Kit-8
References
Abe S, Ochi H, Takahashi Y, Ishijima SA, Osumi M, Yamaguchi H (2000) Characteristic biological effects of itraconazole on L929 fibroblasts and their cell membrane. J Infect Chemother 6(1):35–40
Barbosa Jr F, Sertorio JT, Gerlach RF, Tanus-Santos JE (2006) Clinical evidence for lead-induced inhibition of nitric oxide formation. Arch Toxicol 80(12):811–816
Born GV (1962) Aggregation of platelets by ADP and its reversal. Nature 194(4832):927–928
Brito FCF, Kummerle AE, Lugnier C, Fraga CAM, Barreiro EJ, Miranda ALP (2010) Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 638(1-3):5–12
Celestini A, Violi F (2007) A review of picotamide in the reduction of cardiovascular events in diabetic patients. Vasc Health Risk Manag 3(1):93–98
Eskandariyan Z, Zadeh ME, Tehrani KHME, Mashayekhi V, Kobarfard F (2014) Synthesis of thioether derivatives of quinazoline-4-one-2-thione and evaluation of their antiplatelet aggregation activity. Arch Pharm Res 37(3):332–339
Guthrie R (2011) Review and management of side effects associated with antiplatelet therapy for prevention of recurrent cerebrovascular events. Adv Ther 28(6):473–482
Jayakumar T, Yang CH, Geraldine P, Yen TL, Sheu JR (2016) The pharmacodynamics of antiplatelet compounds in thrombosis treatment. Expert Opin Drug Met 12(6):615–632
Khalid W, Badshah A, Khan AU, Nadeem H (2018) Synthesis, characterization, molecular docking evaluation, antiplatelet and anticoagulant actions of 1,2,4 triazole hydrazone and sulphonamide novel derivatives. Chem Cent J 12(1):11
Li GL, Wang X, Meng X, Lin YB, Li X, Liu XJ (2015) Design, synthesis of novel N, N'-bis-(halogenophenyl)-4-methoxybenzene-1, 3-disulfonamides and evaluation of their anti-platelet aggregation activity. Acta Pharmaceutica Sinica 50(2):185–190
Liu XJ, He X, Shi CL, Meng J, Shao YL, Si HQ, Hu T (2011) Synthesis and in vitro activities on anti-platelet aggregation of N, N’-di(2-substituted-phenyl)-4-methoxyisophthalamides and benzene-1, 3-disulfonamides. Chin Chem Lett 22(10):1139–1142
Liu XJ, Wang CQ, Meng J, Shi XX, Yan YN, Liu XG (2017) Design, synthesis and biological evaluation of 4-methoxy diaryl isophthalates as antiplatelet agents. Med Chem Res 1:1–9
Liu XJ, Wang SQ, Zhang J, Zhang FX, Li GZ, Wang BJ, Shao YL, Zhang LG, Fang L, Cheng MS (2006) Design, synthesis, and activities of novel derivatives of isophthalamide and benzene-1,3-disulfonamide. Chem Res Chin U 22(3):356–359
Liu XJ, Wang Y, Liu LL, Chen GL (2018) Synthesis and in vitro activities on anti-platelet aggregation of 4-methoxyisophthalamides. Med Chem Res 27(8):1971–1983
Liu XJ, Shi TE, Wang X, Wei TT, Meng X (2015) Synthesis and the evaluations in vitro antiplatelet aggregation activitiesof 4-ethoxyisophthalamides. Cardiovasc Hematol Agents Med Chem 13:124–128
Liu XJ, Shi XX, Zhong YL, Liu N, Liu K (2012) Design, synthesis and in vitro activities on anti-platelet aggregation of 4-methoxybenzene-1,3-isophthalamides. Bioorg Med Chem Lett 22(21):6591–6595
Mclewee N, Archer T, Wills R, Mackin A, Thomason J (2017) Effects of aspirin dose escalation on platelet function and urinary thromboxane and prostacyclin levels in normal dogs. Pharmacol Therapeut 41(1):1–8
Metil DS, Sampath A, Reddy CVR, Bandichhor R (2018) Synthesis and characterization of potential related substances of the antiplatelet agent clopidogrel bisulfate. Chemistryselect 3(1): 100–104
Mirfazli SS, Kobarfard F, Firoozpour L, Asadipour A, Esfahanizadeh M, Tabib K, Shafiee A, Foroumadi A (2014) N-Substituted indole carbohydrazide derivatives: synthesis and evaluation of their antiplatelet aggregation activity. Daru 22(1):65
Peng JJ, Zhao LF, Wang LL, Chen H, Qiu YG, Wang J, Yang HY, Liu J, Liu H (2018) Design, synthesis, and biological evaluation of 2-(phenoxyaryl)-3-urea derivatives as novel P2Y1 receptor antagonists. Eur J Med Chem 158:302–310
Pogliani E, Milani M (1996) Safety and efficacy of picotamide, a dual anti-thromboxane agent, in patients with thrombocytosis and a previous thromboembolic event: a 1-year observational study. J Int Med Res 24(3):311
Reddy MVB, Tsai WJ, Qian K, Lee KH, Wu TS (2011) Structure–activity relationships of chalcone analogs as potential inhibitors of ADP-and collagen-induced platelet aggregation. Bioorg Med Chem 19(24):7711–7719
Sharma V, Jaiswal PK, Kumar S, Mathur M, Swami AK, Yadav DK, Chaudhary S (2018) Discovery of Aporphine analogues as potential antiplatelet and antioxidant agents: design, synthesis, structure–activity relationships, biological evaluations, and in silico molecular docking studies. Med Chem 13(17):1817–1832
Siwek A, Staczek P, Stefanska J (2011) Synthesis and structure–activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur J Med Chem 46(11):5717–5726
Wang Y, Wang X, Chen X, Liu XJ (2018) Synthesis and in vitro anti-platelet aggregation activities of 2-methoxy-5-arylamido-N-(pyridin-3-yl-methyl)benzamides. Arch Pharm 352:1–12
Xiong JW, Xiao H, Zhang ZX (2007) An experimental research on different detection conditions between MTT and CCK-8. Acta Laser Biol Sin 16(5):559–562
Yip S, Benavente O (2011) Antiplatelet agents for stroke prevention. Neurotherapeutics 8(3):475–487
Acknowledgements
The authors grate to the National Science Foundation of China (11341014) & the Committee of Science and Technology of Tianjin of China (15JCZDJC33100) for the financial supports and Shenyang Pharmaceutical University of China for running platelet aggregation assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Liu, X., Qiu, k. et al. N, N’-disubstitutedphenyl-4-ethoxyl benzene-1, 3-disulfonamides: design, synthesis, and evaluation of anti-platelet aggregation activity. Med Chem Res 28, 1388–1401 (2019). https://doi.org/10.1007/s00044-019-02379-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02379-5