Abstract
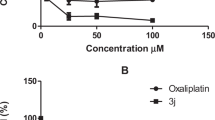
A series of triazolo-lapatinib hybrids were synthesized via copper (II)-oxide nanoparticle (Cu2O-NP)-catalyzed azide-alkyne cycloaddition. The ability of these compounds to reduce the viability of breast cancer SKBR3 and SUM159 cells and stem cell-like KG-1a leukemia cells was subsequently evaluated. Compared with lapatinib, compounds 6c–f were more potent than lapatinib against the three cell lines. Next, the toxicity of compounds 6c–f was assessed in zebrafish. Compound 6d had comparable toxicity with lapatinib at 200 μM (mortality rate = 10 vs. 10%), and compound 6e had lower toxicity than lapatinib at 200 μM (mortality rate = 0 vs. 10%). Thus, compound 6e is a promising lead compound worthy of further investigation.





Similar content being viewed by others
References
Bamford MJ, Dean DK (2004) Wilson DM. 4-(4-(heterocyclylalkoxy)phenyl)-1-(heterocyclyl-carbonyl)piperidine derivatives and related compounds as histamine H3 antagonists for the treatment of neurological diseases such as alzheimer’s. WO patent 89373
Bonnet D, Ilien B, Galzi JL, Riche S, Antheaune C, Hibert M (2006) A rapid and versatile method to label receptor ligands using “click” chemistry: Validation with the muscarinic M1 antagonist pirenzepine. Bioconjug Chem 17:1618–1623
Hegde PS, Rusnak D, Bertiaux M, Alligood K, Strum J, Gagnon R, Gilmer TM (2007) Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther 6:1629–1640
Hein CD, Liu XM, Wang D (2008) Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res 25:2216–2230
Kim JW, Kim H, Im S, Kang S, Hur HS, Yoon Y, Oh D, Kim JH, Lee DS, Kim T, Bang Y (2008) The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett 272:296–306
Lacerda L, Pusztai L, Woodward WA (2010) The role of tumor initiating cells in drug resistance of breast cancer: Implications for future therapeutic approaches. Drug Resist Update 13:99–108
Li X, Lewis MT, Huang J, Gutierrez C, Osbome CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Moses JE, Moorhouse AD (2007) The growing applications of click chemistry. Chem Soc Rev 36:1249–1262
Quesada E, Taylor RJK (2005) One-pot conversion of activated alcohols into terminal alkynes using manganese dioxide in combination with the Bestmann–Ohira reagent. Tetrahedron Lett 36:6473–6476
Ramanadham JP, Bhujanga Rao AKS, Nannapaneni VC (2010) A novel process for the preparation of lapatinib and its pharmaceutically acceptable salts. WO patent 061400
Rasheed ZA, Kowalski JB, Smith BD, Matsui W (2011) Concise Review: Emerging Concepts in Clinical Targeting of Cancer. Stem Cells Stem Cells 29:883–887
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
So JY, Lin JJ, Wahler J, Liby KT, Sporn MB, Suh N (2014) A synthetic triterpenoid CDDO-Im inhibits tumorsphere formation by regulating stem cell signaling pathways in triple-negative breast cancer. PLos One 9:107616–107626
Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K et al. (2004) A unique structure for epidermal growth receptor bound to GW 572016 (1apatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res 64:6652–6659
Zhu W, Ma D (2004) Synthesis of aryl azides and vinyl azides via proline-promoted CuI-catalyzed coupling reactions. Chem Commun 35:888–889
Zhang Z, Dong C, Yang C, Hu D, Long J, Wang L, Li H, Chen Y, Kong D (2010) Stabilized copper(I) oxide nanoparticles catalyze azide-alkyne click reactions in water. Adv Synth Catal 352:1600–1604
Funding
This work was supported by the Natural Science Foundation of Tianjin (No. 16JCQNJC13800 to CGB), National Science & Technology Pillar Program (No. 2015BAI12B15 to ZST), and Higher Learning Basic Scientific Research Business Funded Projects of Tianjin Medical University of Tianjin (No. 2016YD03 to YHS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shi, Y., Zhang, W., Li, L. et al. Design and synthesis of novel triazolo-lapatinib hybrids as inhibitors of breast cancer cells. Med Chem Res 27, 2437–2445 (2018). https://doi.org/10.1007/s00044-018-2247-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2247-0