Abstract
Two series of 16 novel aminoalkylated chalcone derivatives 2a–h and 3a–h were synthesized from 2′-hydroxy-3,4,4′,6′-tetramethoxychalcone (1) through extending alkoxy side chain at the 2′-position, and introducting amine hydrogen bond receptor at the end of the side chain. Their in vitro antiproliferative activities were evaluated on a panel of three human cell lines (Hela, HCC1954, and SK-OV-3) by CCK-8 assay. The results showed that all the target compounds, except compound 3e, exhibited moderate to potent antiproliferative activities against these three human cancer cells with the IC50 values of 6.78–64.45 μmol/L, in particular compounds 2g (on Hela cells), 2c (on HCC1954 cells), and 2c, 2d (on SK-OV-3 cells) possess IC50 values below 10 μmol/L. It showed the introduction of aminoalkyl moiety at 2′-O-position of chalcone 1 resulted to produce the desired effect of increasing the antiproliferative activities, and the distance between the amino groups and chalcone moiety plays an important role, the optimal number of methylene units is two-carbon spacer.
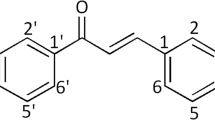
Graphical abstract
A series of 16 novel aminoalkylated chalcones were synthesized and their antiproliferative acivities on three human cancer cells were evaluated.



Similar content being viewed by others
References
Abay ET, Bonnet SL, de Kock C, Kendrekar P, Noreljaleel AEM, Wilhelm A, van der Wiesner L, Westhuizen JH, Swart KJ (2015) Syntheses and in vitro antiplasmodial activity of aminoalkylated chalcones and analogues. J Nat Prod 78:1848–1856
Abd-Rahman N, Mai CW, Kang YB, Pichika MR, Yaeghoobi M (2014) Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur J Med Chem 77:378–387
Balakishan G, Kamal A, Janaki RM, Pal-Bhadra M, Ramakrishna G, Raju P, Viswanath A (2010) Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorg Med Chem Lett 20:4865–4869
Bandeira Graziele D, Evangelista Fernanda CG, Maralice O, Silva Marina G (2016) Synthesis and in vitro evaluation of novel triazole/azide chalcones. Med Chem Res 26:27–43
Bao YM, Li KJ, Ma JG, Sun XD, Yang PW, Zou L, Zhang SX (2008) Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: synthesis, SAR analysis, and biological activity. Bioorg Med Chem 16(15):7127–7132
Barbic M, Heilmann J, Jurgenliemk G, Vogel S (2010) Synthesis, cytotoxicity, antioxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur J Med Chem 45(6):2206–2213
Bonesi M, Deguin B, Loizzo MR, Menichini F, Tillequim F, Tundis R (2008) In vitro biological evaluation of novel 7-O-dialkylaminoalkyl cytotoxic pectolinarigenin derivaties against a panel of human cancer cell lines. Bioorg Med Chem Lett 18:5431–5434
Blanc M, Boccard J, Boumendjel A, Carrupt PA, Choisnard L, Dumontet C, Geze A, Matera EL, Nicolle E, Wouessidjewe D (2008) Antimitotic and antiproliferative activitis of chalcones: forward structure-activity relationship. J Med Chem 51:2307–2310
Campbell A, Do T, Hofmann E, Higginbottom G, Hauser Q, Kline R, Ma LL, Paula S, Snider L, Webster J (2016) Hydroxylated chalcones with dual properties: xanthine oxidase inhibitors and radical scavengers. Bioorg Med Chem 24:578–587
Chen BQ, Liu YM, Shi YP, Wang P, Xuan LN, Zhang K, Zhu T (2015) Synthesis and in vitro antiproliferative activity of novel benzisoselenazolone derivatives. Med Chem Res 24:543–552
Chen H, Lu SH, Shen AJ, Song YL, Tian W, Wang MP, Yang C, Zhang L, Zhang M, Zheng CH, Zhu J, Zhou YJ (2016) Structural modification of luteolin from Flos Chrysanthemi leads to increased tumor cell growth inhibitory activity. Bioorg Med Chem Lett 26:3464–3467
Detsi A, Hadjipavlou –Litina D, Kontogiorgic CA, Kefalas P, Majdalan M (2009) Natural and synthetic 2′-htdroxy-chalcones and aurones: synthesis, characterization and evaluation of the antioxidsant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem 17:8073–8085
Dominy BW, Feeney PJ, Lipinski CA, Lombardo F (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–25
Dong XW, Du LL, Hu YZ, Liu T, Pan ZC, Yang B (2010) Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur J Med Chem 45:3986–3992
Dong LP, Nguyen VS, Wang QA, Wang SC (2015) The first total synthesis of sophoflavescenol, flavenochromane C and citrusinol. Eur J Org Chem 2015(10):2297–2302
EI-Melige S, Kamal AM, Taher AT, Youssef A (2017) Design, synthesis and cytotoxic activity of certain novel chalcone analogous compounds. Eur J Med Chem 126:52–60
Emami S, Mirzaei H (2016) Recent advances of cytotoxic chalconoids targeting tubulin polymerization: synthesis and biological activity. Eur J Med Chem 121:610–639
Fernandes E, Feritas M, Ribeiro D (2014) Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur J Med Chem 86:153–164
Huang XQ, Liu HR, Liu XJ, Liu WK, Lou DH, Wang QA (2014) Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives of flavokawain B. Bioorg Med Chem Lett 24:4749–4753
Kuppuswami BK, Manogaran P, Narasimha KKG, Raghavan S, Venkatraman G (2015) Synthesis and anticancer activity of chalcones derived from vanillin and isovanillin. Med Chem Res 24:4157–4165
Lipinski CA (2004) Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1(4):337–341
Li Y, Li W, Nguyen VS, Wang QA (2017) Synthesis of citrus polymethoxyflavonoids and their antiproliferative activities on Hela cells. Med Chem Res 26(7):1585–1592
Nguyen VS, Shi L, Wang QA, Wang SC (2017) Synthesis of icaritin and β-anhydroicaritin Mannich base derivatives and their cytotoxic activities on three human cancer cell lines. Anticancer Agents Med Chem 17(1):137–142
Acknowledgements
We thank the National Natural Science Foundation of China (No. J1210040) and Education Department of Hubei Province Science and Technology Research Project of China (No. Q20162803) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, C., Wang, G., Li, X. et al. Synthesis and antiproliferative activity of aminoalkylated chalcones on three human cancer cells. Med Chem Res 27, 972–979 (2018). https://doi.org/10.1007/s00044-017-2120-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2120-6