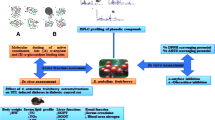
Abstract
Profiling the polyphenols in the methanol extract from the bark of Albizia harveyi was performed by HPLC–PDA–ESI–MS/MS analysis. The phytochemical analysis identified 39 compounds, the majority of them were flavan-3-ol derivatives and condensed tannins. Total phenolic content, determined by the Folin–Ciocalteu method amounted to 489 mg gallic acid equivalents/g extract. The extract showed promising antioxidant activities with an EC50 of 3.6 µg/mL and 18.32 mM FeSO4 equivalent/mg extract in radical scavenging assay and ferric reducing antioxidant power assays, respectively. The hepatoprotective potential of the extract in rats was determined in vivo in a d-galactosamine-induced liver toxicity model. A dose of 100 mg/kg (body weight) of the bark extract reduced levels of aspartate aminotransferase, gamma-glutamyltransferase and total bilirubin by 35.7, 65.3, and 23.8% (p < 0.05), respectively whereas glutathione was increased by 59.1%. These effects were similar to silymarin which was used as positive control. The extract (100 mg/kg (body weight) mediated a substantial antidiabetic response in streptozotocin-induced diabetic rats manifested by a significant reduction in serum glucose and lipid peroxides and significant increase of serum insulin. Docking of d-(+) catechin and the dimer (epi)catechin-(epi)catechin into the active site of the enzymes human pancreatic α-amylase, maltase-glucoamylase, and aldol reductase revealed that these enzymes may be possible targets via which, the studied Albizia harveyi extract could exert its antidiabetic effect.












Similar content being viewed by others
References
Abbas S, Wink M (2009) Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med 75:216–221
Ahmed D, Kumar V, Verma A, Gupta PS, Kumar H, Dhingra V, Mishra V, Sharma M (2014) Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizia lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC 14:1
Arumugam G, Manjula P, Paari N (2013) A review: anti diabetic medicinal plants used for diabetes mellitus. J Acute Dis 2:196–200
Balisteri W, Shaw L (1987) Liver function. In Tietz NW (ed) Fundamentals of clinical chemistry, 3rd edn. WB Saunders, Philadelphia, p 729–761
Bergantin C, Maietti A, Cavazzini A, Pasti L, Tedeschi P, Brandolini V, Marchetti N (2017) Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus). J Funct Foods 33:94–102
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Brayer GD, Luo Y, Withers SG (1995) The structure of human pancreatic α‐amylase at 1.8 Å resolution and comparisons with related enzymes. Protein Sci. 4:1730–1742
Brenan JPM (1970) Flora Zambesiaca, 3, 1. Crown Agents for Oversea, London
de Souza LM, Cipriani TR, Iacomini M, Gorin PA, Sassaki GL (2008) HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J Pharm Biomed Anal 47:59–67
Del Rio D, Stewart AJ, Mullen W, Burns J, Lean ME, Brighenti F, Crozier A (2004) HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric. Food Chem. 52:2807–2815
Dixit AK, Misra LN (1997) Macrocyclic budmunchiamine alkaloids from Albizia lebbeck. J Nat Prod 60:1036–1037
Doumas BT, Watson WA, Biggs HG (1997) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 258:21–30
El-Mousallamy AMD (1998) Leaf flavonoids of Albizia lebbeck. Phytochemistry 48:759–761
Fanfrlík Ji, Ruiz FX, Kadlčíková A, Řezáč J, Cousido-Siah A, Mitschler A, Haldar S, Lepšík M, Kolář MH, Majer P (2015) The effect of halogen-to-hydrogen bond substitution on human aldose reductase inhibition. ACS Chem Biol 10:1637–1642
Gonzalez-Manzano S, Santos-Buelga C, Parez-Alonso JJ, Rivas-Gonzalo JC, Escribano-Bailan MT (2006) Characterization of the mean degree of polymerization of proanthocyanidins in red wines using liquid chromatography-mass spectrometry (LC-MS). J Agric Food Chem 54:4326–4332
Gordon C, Yates A, Davies D (1985) Evidence for a direct action of exogenous insulin on the pancreatic islets of diabetic mice: islet response to insulin pre-incubation. Diabetologia 28:291–294
Guimaraes R, Barros L, Duenas M, Carvalho AM, Queiroz MJR, Santos-Buelga C, Ferreira IC (2013) Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem 141:3721–3730
Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C (2010) Characterization of phenolic composition in Lamiaceae species by LC-ESI-MS/MS. J Agric Food Chem 58:10576–10581
Kang TH, Jeong SJ, Kim NY, Higuchi R, Kim YC (2000) Sedative activity of two flavonol glycosides isolated from the flowers of Albizia julibrissin Durazz. J Ethnopharmacol 71:321–323
Keppler D, Lesch R, Reutter W, Decker K (1968) Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol 9:279–290
Kokila K, Priyadharshini SD, Sujatha V (2013) Phytopharmacological properties of Albizia species: a review. Int J Pharm Pharm Sci 5:70–73
Lee MS, Kerns EH (1999) LC/MS applications in drug development. Mass Spectrom Rev 18:187–279
Lin L-Z, Harnly JM (2007) A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J Agric Food Chem 55:1084–1096
Maritim A, Sanders a, Watkins rJ (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Mena P, Calani L, Dall’Asta C, Galaverna G, García-Viguera C, Bruni R, Crozier A, Del Rio D (2012) Rapid and comprehensive evaluation of (poly) phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 17:14821–14840
Michael, J, James MC, Vinay K (2000) The pancreas. In: Vinay K, Tucker C, Ramzi Sc (eds) Robbins and Cotran Pathalogic basis of disease, 6th edn. Harcourt publisher, San Diego, CA, p 902–929
Mohamed TK, Nassar MI, Gaara AH, El-Kashak WA, Brouard I, El-Toumy SA (2013) Secondary metabolites and bioactivities of Albizia anthelmintica. Pharmacogn Res 5:80
Mooradian AD, Thurman JE (1999) Drug therapy of postprandial hyperglycaemia. Drugs 57:19–29
Murray R (1984) Alanine aminotransferase. In: Kaplan A et al. (eds) Clin Chem. The C.V. Mosby Co. Si louil. Tronto, Princeton, pp 1088–1090.
Nibret E, Ashour ML, Rubanza CD, Wink M (2010) Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother Res 24:945–947
Nichols BL, Avery S, Sen P, Swallow DM, Hahn D, Sterchi E (2003) The maltase-glucoamylase gene: common ancestry to sucrase-isomaltase with complimentary starch digestion activities. Proc Natl Acad Sci 100:1432–1437
Nichols BL, Quezada-Calvillo R, Robayo-Torres CC, Ao Z, Hamaker BR, Butte NF, Marini J, Jahoor F, Sterchi EE (2009) Mucosal maltase-glucoamylase plays a crucial role in starch digestion and prandial glucose homeostasis of mice. J Nutr 139:684–690
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omar HS, El-Beshbishy HA, Moussa Z, Taha KF, Singab ANB (2011) Antioxidant activity of Artocarpus heterophyllus Lam.(Jack Fruit) leaf extracts: remarkable attenuations of hyperglycemia and hyperlipidemia in streptozotocin-diabetic rats. Sci World J 11:788–800
Parola M, Robino G (2001) Oxidative stress-related molecules and liver fibrosis. J Hepatol 35:297–306
Phillips M, Cataneo RN, Cheema T, Greenberg J (2004) Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta 344:189–194
Quezada-Calvillo R, Sim L, Ao Z, Hamaker BR, Quaroni A, Brayer GD, Sterchi EE, Robayo-Torres CC, Rose DR, Nichols BL (2008) Luminal starch substrate “brake” on maltase-glucoamylase activity is located within the glucoamylase subunit. J Nutr 138:685–692
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Ramana KV (2011) Aldose reductase: new insights for an old enzyme. Biomol Concept 2:103–114
Ren L, Qin X, Cao X, Wang L, Bai F, Bai G, Shen Y (2011) Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2:827–836
Rukunga GM, Waterman PG (1996) Spermine alkaloids from Albizia schimperiana. Phytochemistry 42:1211–1215
Rydberg EH, Sidhu G, Vo HC, Hewitt J, Cote HC, Wang Y, Numao S, Macgillivray RT, Overall CM, Brayer GD (1999) Cloning, mutagenesis, and structural analysis of human pancreatic [alpha]-amylase expressed in Pichia pastoris. Protein Sci 8:635–643
Setshogo MP (2005) Preliminary checklist of the plants of Botswana. Southern African Botanical Diversity Network Report No. 37. SABONET, Pretoria and Gaborone
Sicree R, Shaw J, Zimmet P (2006) Diabetes and impaired glucose tolerance. Diabetes Atlas, 3rd edn. International Diabetes Federation, Belgium
Simsek M, Quezada-Calvillo R, Ferruzzi MG, Nichols BL, Hamaker BR (2015) Dietary phenolic compounds selectively inhibit the individual subunits of maltase-glucoamylase and sucrase-isomaltase with the potential of modulating glucose release. J Agric Food Chem 63:3873–3879
Sobeh M, ElHawary EA, Peixoto H, Labib RM, Handoussa H, Swilam N, El-Khatib AH, Sharapov F, Mahmoud T, Krstin S, Linscheid MW, Singab ANB, Wink M Ayoub N (2016a) Identification of phenolic secondary metabolites from Schotia brachypetala Sond.(Fabaceae) and demonstration of their antioxidant activities in Caenorhabditis elegans. PeerJ. doi:10.7717/peerj.2404
Sobeh M, Mamadalieva NZ, Mohamed T, Krstin S, Youssef FS, Ashour ML, Azimova SS, Wink M (2016b) Chemical profiling of Phlomis thapsoides (Lamiaceae) and in vitro testing of its biological activities. Med Chem Res 25:2304–2315
Sobeh M, Mahmoud MF, Abdelfattah MAO, El-Beshbishy HA, El-Shazly AM, Wink M (2017) Hepatoprotective and hypoglycemic effects of a tannin rich extract from Ximenia americana var. caffra root. Phytomedicine 33:36–42
Spitaler M, Graier W (2002) Vascular targets of redox signalling in diabetes mellitus. Diabetologia 45:476–494
Stefek M, Soltesova Prnova M, Majekova M, Rechlin C, Heine A, Klebe G (2015) Identification of novel aldose reductase inhibitors based on carboxymethylated mercaptotriazinoindole scaffold. J Med Chem 58:2649–2657
Szasz G, Persijn JPE,C (1974) Kinetic method for quantitative determination of gammaglutamyl transpeptidase. Z Klin Chem Klin Biochem 12:228
Tala VRS, Candida da Silva V, Rodrigues CM, Nkengfack AE, Campaner dos Santos L, Vilegas W (2013) Characterization of proanthocyanidins from Parkia biglobosa (Jacq.) G. Don. (Fabaceae) by flow injection analysis-electrospray ionization ion trap tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectrometry. Molecules 18:2803–2820
Timberlake JR, Childes SL (2004) Biodiversity of the four corners area: technical reviews Volume Two (Chapter 5-15) Appendix 5-1: Plant Checklist. Occas Publ Biodivers 15:179
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Williams LK, Li C, Withers SG, Brayer GD (2012) Order and disorder: differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J Med Chem 55:10177–10186
Yabe-Nishimura C (1998) Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev 50:21–34
Yilmazer-Musa M, Griffith AM, Michels AJ, Schneider E, Frei B (2012) Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J Agric Food Chem 60:8924–8929
Young DS (1995) Effects of drugs on clinical laboratory tests. The American Association for Clinical Chemistry. Young, Washington, DC
Youssef FS, Ashour ML, Ebada SS, Sobeh M, El-Beshbishy HA, Singab AN, Wink M (2017) Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 69: 733–742
Youssef FS, Ashour ML, Sobeh M, El-Beshbishy HA, Singab AN, Wink M (2016) Eremophila maculata- Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine 23:1484–1493
Zheng L, Zheng J, Zhao Y, Wang B, Wu L, Liang H (2006) Three anti-tumor saponins from Albizia julibrissin. Bioorg Med Chem Lett 16:2765–2768
Acknowledgements
The authors would like to thank Dr. C. D. Rubanza (Tanzania Forestry Research Institute, Shinyanga, Tanzania) for plant collection and Dr. B. Wetterauer (IPMB) for his help in collecting LC-MS data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests
Rights and permissions
About this article
Cite this article
Sobeh, M., Mahmoud, M.F., Abdelfattah, M.A.O. et al. Albizia harveyi: phytochemical profiling, antioxidant, antidiabetic and hepatoprotective activities of the bark extract. Med Chem Res 26, 3091–3105 (2017). https://doi.org/10.1007/s00044-017-2005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2005-8