Abstract
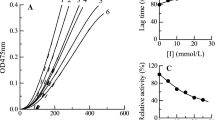
In this paper, the inhibitory effects of 4-chlorocinnamaldehyde on mushroom tyrosinase were investigated. The results showed that 4-chlorocinnamaldehyde had a significant inhibitory effect on monophenolase and diphenolase in tyrosinase. 4-Chlorocinnamaldehyde could obviously prolong the lag phase of monophenolase and it decreased the steady-state rate of both monophenolase and diphenolase. The IC50 values were found to be 0.07 and 0.3 mM for monophenolase and diphenolase, respectively. The kinetic studies showed that the inhibitory mechanism of mushroom tyrosinase by 4-chlorocinnamaldehyde was the reversible competitive inhibition. The inhibition constant (K I) was measured to be 0.17 mM. The experimental research demonstrated that 4-chlorocinnamaldehyde was an effective tyrosinase inhibitor and the research results may offer the theoretical basis for designing and synthesizing safer and more efficient tyrosinase inhibitors in future.






Similar content being viewed by others
References
Alijanianzadeh M, Saboury AA, Ganjali MR, Hadi-Alijanvand H, Moosavi-Movahedi AA (2012) The inhibitory effect of ethylenediamine on mushroom tyrosinase. Int J Biol Macromol 50:573–577
Ashraf Z, Rafiq M, Seo S-Y, Kwon K-S, Babar MM, Zaidi N-u-SS (2015) Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur J Med Chem 98:203–211
Bao K, Dai Y, Zhu Z-B, Tu F-J, Zhang W-G, Yao X-S (2010) Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorgan Med Chem 18:6708–6714
Chai W-M, Liu X, Hu Y-H, Feng H-L, Jia Y-L, Guo Y-J, Zhou H-T, Chen Q-X (2013) Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int J Biol Macromol 57:151–155
Chan C-F, Lai S-T, Guo Y-C, Chen M-J (2014) Inhibitory effects of novel synthetic methimazole derivatives on mushroom tyrosinase and melanogenesis. Bioorgan Med Chem 22:2809–2815
Chen Q-X, Song K-K, Qiu L, Liu X-D, Huang H, Guo H-Y (2005) Inhibitory effects on mushroom tyrosinase by p-alkoxybenzoic acids. Food Chem 91:269–274
Chen XX, Zhang J, Chai WM, Feng HL, Xiang ZH, Shen DY, Chen QX (2013) Reversible and competitive inhibitory kinetics of amoxicillin on mushroom tyrosinase. Int J Biol Macromol 62:726–733
Georgiev L, Chochkova M, Totseva I, Seizova K, Marinova E, Ivanova G, Ninova M, Najdenski H, Milkova T (2012) Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med Chem Res 22:4173–4182
Han P, Chen C-Q, Zhang C-L, Song K-K, Zhou H-T, Chen Q-X (2008) Inhibitory effects of 4-chlorosalicylic acid on mushroom tyrosinase and its antimicrobial activities. Food Chem 107:797–803
Hu Y-H, Zhuang J-X, Yu F, Cui Y, Yu W-W, Yan C-L, Chen Q-X (2016) Inhibitory effects of cefotaxime on the activity of mushroom tyrosinase. J Biosci Bioeng 121(4):385–389
Jiménez M, Chazarra S, Escribano J, Cabanes J, García-Carmona F (2001) Competitive inhibition of mushroom tyrosinase by 4-substituted benzaldehydes. J Agric Food Chem 49:4060–4063
Khan MTH (2007) Molecular design of tyrosinase inhibitors: a critical review of promising novel inhibitors from synthetic origins. Pure Appl Chem 79(12):2277–2295
Liu X, Jia Y-l, Chen J-w, Liang G, Guo H-y, Hu Y-h, Shi Y, Zhou H-T, Chen Q-X (2015) Inhibition effects of benzylideneacetone, benzylacetone, and 4-phenyl-2-butanol on the activity of mushroom tyrosinase. J Biosci Bioeng 119(3):275–279
Qiu L, Chen Q-H, Zhuang J-X, Zhong X, Zhou J-J, Guo Y-J, Chen Q-X (2009) Inhibitory effects of α-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase. Food Chem 112:609–613
Radhakrishnan S, Shimmon R, Conn C, Baker A (2015) Integrated kinetic studies and computational analysis on naphthyl chalcones as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 25:4085–4091
Wang Q, Shi Y, Song K-K, Guo H-Y, Qiu L, Chen Q-X (2004) Inhibitory effects of 4-halobenzoic acids on the diphenolase and monophenolase activity of mushroom tyrosinase. Protein J 23(5):303–308
Xie J, Dong H, Yu Y, Cao S (2016) Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on mushroom tyrosinase: insights from fluorescence, 1H NMR titration and molecular docking studies. Food Chem 190:709–716
Xing R, Wang F, Dong L, Zheng AP, Wang L, Su W-J, Lin T (2016) Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem 197:205–211
Yi W, Cao R, Peng W, Wen H, Yan Q, Zhou B, Ma L, Song H (2010) Synthesis and biological evaluation of novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors. Eur J Med Chem 45:639–646
Zhu Y-J, Zhou H-T, Hu Y-H, Tang J-Y, Su M-X, Guo Y-J, Chen Q-X, Liu B (2011) Antityrosinase and antimicrobial activities of 2-phenylethanol,2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem 124:298–302
Acknowledgements
This work was supported by the Innovation Team of Scientific Research Platform in Anhui Universities, the Key Laboratory of Bioresource Protection and Utilization of Anhui Province, the Key Laboratory of Biotic Environment and Ecological Safety of Anhui Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Si, H., Wang, X., Li, L. et al. Inhibitory effects of 4-chlorocinnamaldehyde on the activity of mushroom tyrosinase. Med Chem Res 26, 1377–1381 (2017). https://doi.org/10.1007/s00044-017-1861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1861-6