Abstract
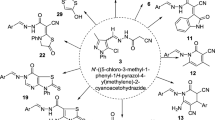
 A series of new hybrid molecules with a hydrazone fragment were synthesized and characterized by Fourier transform-infrared spectroscopy, nuclear magnetic resonance, mass spectrometry, and elemental analysis. The nuclear magnetic resonance spectra of the hydrazones 4a–c showed exchange of syn and antiperiplanar conformers around the amide bond, the more stable being the antiperiplanar one. The nuclear Overhauser effect spectroscopy (NOESY) spectra confirm E configuration around C=N bond. The tested compounds exhibited concentration-dependent cytotoxic effects against human tumor cell lines in a micromolar range (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay). Hydrazone 4a proved to be the most potent antiproliferative agent of the series. Within the antioxidant screening 8b exhibited the highest radical scavenging activity (% RSA max) and the largest rate constant for the reaction with 2,2-diphenyl-1-picrylhydrazyl.
A series of new hybrid molecules with a hydrazone fragment were synthesized and characterized by Fourier transform-infrared spectroscopy, nuclear magnetic resonance, mass spectrometry, and elemental analysis. The nuclear magnetic resonance spectra of the hydrazones 4a–c showed exchange of syn and antiperiplanar conformers around the amide bond, the more stable being the antiperiplanar one. The nuclear Overhauser effect spectroscopy (NOESY) spectra confirm E configuration around C=N bond. The tested compounds exhibited concentration-dependent cytotoxic effects against human tumor cell lines in a micromolar range (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay). Hydrazone 4a proved to be the most potent antiproliferative agent of the series. Within the antioxidant screening 8b exhibited the highest radical scavenging activity (% RSA max) and the largest rate constant for the reaction with 2,2-diphenyl-1-picrylhydrazyl.




Similar content being viewed by others
References
Abdel-Aziz HA, Aboul-Fadl T, Abdul-Rahman MAl-Obaid, Ghazzali M, Al-Dhfyan A, Contini A (2012) Synthesis and pharmacophoric model building of novel substituted nicotinic acid hydrazones with potential antiproliferative activity. Arch Pharm Res 35(9):1543–1552
Abdel-Aziz HA, Elsaman T, Al-Dhfyan A, Attia MI, Al-Rashood KA, Al-Obaid ARM (2013) Synthesis and anticancer potential of certain novel 2-oxo-N-(2-oxoindolin-3-ylidene)-2H-chromene-3-carbohydrazides. Eur J Med Chem 70:358–363
Abdel-Aziz AH, Ghabbour HA, Eldehna WM, Qabeel MM, Hoong-Kun Fun (2013) Synthesis, crystal structure, and biological activity of cis/trans amide rotomers of (Z)-N-(2-oxoindolin-3-ylidene)formohydrazide. Eur J Med Chem 70:358–363
Alam MS, Lee D-Ung (2015) Quantum-chemical studies to approach the antioxidant mechanism of nonphenolic hydrazone Schiff base analogs: synthesis, molecular structure, Hirshfeld and density functional theory analyses. Bull Korean Chem Soc 36:682–691
Asif M (2014) Pharmacologically potentials of hydrazonone containing compounds: a promising scaffold. Int J Advanced Chem 2(2):85–103
Azizmohammadi M, Khoobi M, Ramazani A, Emami S, Zarrin A, Firuzi O, Miri R, Shafiee A (2013) 2H-chromene derivatives bearing thiazolidine-2,4-dione, rhodanine or hydantoin moieties as potential anticancer agents. Eur J Med Chem 59:15–22
Becker EM, Lovejoy DB, Greer JM, Watts R, Richardson DesR (2003) Identification of the di-pyridyl ketone isonicotinoyl hydrazone (PKIH) analogues as potent iron chelators and anti-tumor agents. British J Pharmacology 138:819–830
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Bubols GB, da Rocha VD, Medina-Remónd A, Poser GL von, Lamuela-Raventos RM, Eifler-Lima VL, Garcia SC (2013) The antioxidant activity of coumarins and flavonoids. Mini-rev Med Chem 13(3):318–334.
Chaston TB, Lovejoy D, Watts RN, Richardson DR (2003) Examination of the anti-proliferative activity of iron chelators: multiple cellular targets and the different mechanism of action of triapine compared to desferrioxamine and the potent PIH analogue 311. Clin Cancer Res 9(1):402–414
Chaston TB, Richardson DR (2003) Iron chelators for the treatment of iron overload disease: relationship between structure, redox activity, and toxicity. Am J Hematology 73:200–210
Congiu C, Onnis V (2013) Synthesis and biological evaluation of novel acylhydrazone derivatives as potential antitumor agents. Bioorg Med Chem 21:6592–6599
De Oliveira KN, Costa P, Santin JR, Mazzambani L, Bürger C, Mora C, Nunesa RJ, de Souzab MM (2011) Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg Med Chem 19:4295–4306
Edward JT, Gauthier M, Chubb FL, Ponka P (1988) Synthesis of new acylhydrazones as iron-chelating compounds. J Chem Eng Data 33:538–540
Ellis S, Kalinowski DS, Leotta L, Huang MLH, Jelfs P, Sintchenko V, Richardson Des R, Triccas JA (2014) Potent antimycobacterial activity of the pyridoxal isonicotinoyl hydrazone analog 2-pyridylcarboxaldehyde isonicotinoyl hydrazone: a lipophilic transport vehicle for isonicotinic acid hydrazide. Mol Pharmacol 85:269–278
El-Tombary AA, El-Hawash SAM (2014) Synthesis, antioxidant, anticancer and antiviral activities of novel quinoxaline hydrazone derivatives and their acyclic C-nucleosides. Med Chem 10(5):521–532
Gaspar A, Martins M, Silva P, Garrido EM, Garrido J, Firuzi O, Miri R, Saso L, Borges F (2010) Phenolic acids and derivatives: evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J Agricultural and Food Chem 58:11273–11280
Gupte A, Mumper RJ (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treatment Reviews 35:32–46
Heber D, Ivanov IC, Karagiosov SK (1995) The vilsmeier reactin in the synthesis of 3-substituted [1]benzopyrano[4,3-b]pyridine-5-ones. J Heterocycl Chem 32(2):505–509
Hermes-Lima M, Nagy E, Ponka P, Schulman HM (1998) The iron chelator pyridoxal isonicotinoyl hydrazone (PIH) protects plasmid pUC-18 DNA against •OH-mediated strand breaks. Free Radical Bio Med 25(8):875–880
Jain PK, Joshi H (2012) Coumarin: chemical and pharmacological profile. J Applied Pharmaceutical Sci 02(06):236–240
Jain J, Kumar Y, Sinha R, Kumar R, Stables J (2011) Menthone aryl acid hydrazones: a new class of anticonvulsants. Medicinal Chem 7:56–61
Jashari A, Imeri F, Ballazhi L, Shabani A, Mikhova B, Drager G, Popovski E, Huwiler A (2014) Synthesis and cellular characterization of novel isoxazolo- and thiazolohydrazinylidene-chroman-2,4-diones on cancer and non-cancer cell growth and death. Bioorg Med Chem 22(9):2655–2661
Ma J, Chen D, Lu K, Wang L, Han X, Zhao Y, Gong P (2014) Design, synthesis, and structure-activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur J Med Chem 86:257–269
Kaplancıklı AZ, Yurttas L, Turan-Zitouni G, Özdemir A, Göger G, Demirci F, Mohsen UA (2014) Synthesis and antimicrobial activity of new pyrimidine-hydrazones. Letters in Drug Design & Discovery 11:76–81
Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M (1999) Mechanistic studies of catechins as antioxidants against radical oxidation. Arch Biochem Biophys 362(1):79–86
Konstantinov SM, Eibl H, Berger MR (1999) BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br J Haematol 107(2):365–380
Kontogiorgis C, Detsi A, Hadjipavlou-Litina D (2012) Coumarin-based drugs: a patent review (2008—present). Expert Opin Ther Patents 22(4):437–454
Lacy A, O’Kennedy R (2004) Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des 10:3797–3811
Lopes AB, Miguez E, Kümmerle AE, Rumjanek VM, Fraga CA, Manssour E, Barreiro J (2013) Characterization of amide bond conformers for a novel heterocyclic template of N-acylhydrazone derivatives. Molecules 18:11683–11704
Lovejoy DB, Richardson DR (2003) Iron chelators as anti-neoplastic agents: current developments and promise of the PIH class of chelators. Curr Med Chem 10:1035–1049
Masesane IB, Yibralign ZY (2012) Reactions of salicylaldehyde and enolates or their equivalents: versatile synthetic routes to chromane derivatives. Beilstein J Org Chem 8:2166–2175
Mohareb RM, Fleita DH, Sakka OK (2011) Synthesis of hydrazide-hydrazone derivatives and their utilization in the synthesis of coumarin, pyridine, thiazole and thiophene derivatives with antitumor activity. Molecules 16:16–27
Mohareb RM, Ibrahim RA, Moustafa HE (2010) Hydrazide-hydrazones in the synthesis of 1,3,4-oxadiazine, 1,2,4-triazine and pyrazole derivatives with antitumor activities. The Open Org Chem J 4:8–14
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1-2(65):55–63
Nasr T, Bondock S, Youns M (2014) Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur J Med Chem 76:539–548
Nikolova-Mladenova B, Halachev N, Iankova R, Momekov G, Ivanov D (2011) Synthesis, characterization and cytotoxic activity of new salicylaldehyde benzoylhydrazone derivatives as potential anti-proliferative agents. Arzneimittelforschung/Drug research 61(12):714–718
Oyaizu M (1986) Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutrition 44:307–315
Rahmani-Nezhad S, Safavi M, Pordeli M, Ardestani S, Khosravani L, Pourshojaei Y, Mahdavi M, Emami S, Foroumadi A, Shafiee A (2014) Synthesis, in vitro cytotoxicity and apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives as potent anti-breast cancer agents. Eur J Med Chem 86:562–569
Rollas S, Küçükgüzel ŞG (2007) Biological activities of hydrazone derivatives. Molecule 12:1910–1939
Sabatié A, Végh D, Loupy A, Floch L (2001) Synthesis of aromatic and heteroaromatic annelated [1,4]diazepines. Arkivoc vi:122–128
Shadnia H, Wright JS (2009) Using molecular strain and aromaticity to create ultraweak C-H bonds and stabilized carbon-centered radicals. J Chem Theory Comput 5(4):1129–1136
Sondhi SM, Dinodia M, Kumar A (2006) Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg Med Chem 14:4657–4663
Stewart WE, Siddall TH (1970) Nuclear magnetic resonance studies of amides. Chem Rev 70(5):517–551
Suslov EV, Korchagina DV, Volcho KP, Salakhutdinov NF (2009) Interaction of salicylaldehyde and crotonaldehyde in the presence of zeolite Csβ, synthesis of heterocyclic compounds using basic zeolite Csβ. Chem Heterocyclic Comp 45(5):560-566.
Taha M, Nor Hadiani Ismail, Waqas Jamil, Sammer Yousuf, Faridahanim Mohd Jaafar, Muhammad Imran Ali, Syed Muhammad Kashif, Ejaz Hussain (2013) Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 18:10912–10929
Thorat BR, Kamat P, Khandekar D, Lele S, Mustapha M, Sawant S, Jadhav R, Kolekar S, Yamgar R, Atram RG (2011) Synthesis and fluorescence study of novel Schiff bases of isoniazide. J Chem Pharm Res 3(6):1109–1117
Thuy LT, Tien HX, Hoang VD, Vu TK (2012) Design, synthesis and in vitro antimalarial evaluation of new quinolinylhydrazone derivatives. Letters in Drug Design & Discovery 9:163–168
Wang Q, Pan Y, Wang J, Peng Q, Luo H, Zheng J (2011) Synthesis and biological activities of substituted N′- benzoylhydrazone derivatives. African J Biotech 10(78):18013–18021
Wiberg KB, Rablen PR, Rush DJ, Keith TA (1995) Amides 3: experimental and theoretical studies of the effect of the medium on the rotational barriers for N,N-dimethylformamide and N,N-dimethylacetamide. J Am Chem Soc 117:4261–4264
Yong-Ling Shi, Min Shi (2007) The synthesis of chromenes, chromanes, coumarins and related heterocycles via tandem reactions of salicylic aldehydes or salicylic imines with α,β-unsaturated compounds. Org Biomol Chem 5:1499–1504
Fu Y, Zhou S, Liu Y, Yang Y, Sun X, Changzheng Li (2014) The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone being involved in topoisomerase IIα inhibition. BioMed Res Int. http://dx.doi.org/10.1155/2014/527042
Acknowledgments
Dedicated to Professor Ivan G. Pojarlieff on the occasion of his 80th birthday. This study was supported by the National Research Fund of Bulgaria (projects UNA-17/2005 and DRNF-02/13/2009) and by the Ministry of Education, Science and Technological Development of Republic of Serbia (Project No. III41018). We appreciate greatly the help with mass spectroscopy of V. Arabadjiev.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Angelova, V.T., Vassilev, N.G., Nikolova-Mladenova, B. et al. Antiproliferative and antioxidative effects of novel hydrazone derivatives bearing coumarin and chromene moiety. Med Chem Res 25, 2082–2092 (2016). https://doi.org/10.1007/s00044-016-1661-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1661-4