Abstract
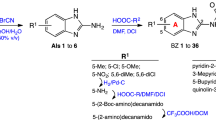
A new series of side chain-modified 4-aminoquinolines were synthesized and screened for in vitro antiplasmodial activity against both chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of Plasmodium falciparum. Among the series, compounds 30 and 31 showed significant inhibition of parasite growth against K1 strain of P. falciparum with IC50 values 0.28 and 0.31 µM, respectively, whereas compounds 34, 35, and 38 exhibited superior activity against K1 strain with IC50 values 0.18, 0.22, and 0.17 µM, respectively, as compared to 0.255 µM for chloroquine (CQ). All the compounds displayed good resistance factor between 1.54 and >34.48 as against 51.0 for CQ. All these analogues were found to form strong complex with hematin and inhibited the β-hematin formation in vitro, suggesting that this class of compounds act on a heme polymerization target. Overall results suggest that present series of compounds appear to be promising for further lead optimization to obtain compounds active against drug-resistant parasites.
Graphical Abstract





Similar content being viewed by others
Abbreviations
- CQ:
-
Chloroquine
- DCC:
-
N,N′-dicyclohexylcarbodiimide
- HOBt:
-
Hydroxybenzotriazole
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
References
Burnett JC, Opsenica D, Sriraghavan K, Panchal RG et al (2007) A refined pharmacophore identifies potent 4-amino-7-chloroquinoline-based inhibitors of the botulinum neurotoxin serotype A metalloprotease. J Med Chem 50:2127–2136. doi:10.1021/jm061446e
Cheruku SR, Maiti S, Dorn A, Scorneaux B, Bhattacharjee AK, Ellis WY, Vennerstrom JL (2003) Carbon isosteres of the 4-aminopyridine substructure of chloroquine: effects on pK(a), hematin binding, inhibition of hemozoin formation, and parasite growth. J Med Chem 46:3166–3169. doi:10.1021/jm030038x
Dalal S, Ragheb DR, Klemba M (2012) Engagement of the S1, S1′ and S2′ subsites drives efficient catalysis of peptide bond hydrolysis by the M1-family aminopeptidase from Plasmodium falciparum. Mol Biochem Parasitol 183:70–77. doi:10.1016/j.molbiopara.2012.02.003
De D, Krogstad FM, Byers LD, Krogstad DJ (1998) Structure-activity relationships for antiplasmodial activity among 7-substituted 4-aminoquinolines. J Med Chem 41:4918–4926. doi:10.1021/jm980146x
Deshpande S (2010) Synthesis, biological evaluation and QSAR studies of novel antimalarial agents PhD Thesis, Jawaharlal Nehru University, New Delhi
Deshpande S, Solomon VR, Katti SB, Prabhakar YS (2009) Topological descriptors in modelling antimalarial activity: N(1)-(7-chloro-4-quinolyl)-1,4-bis(3-aminopropyl)piperazine as prototype. J Enzyme Inhib Med Chem 24:94–104. doi:10.1080/14756360801915377
Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG (1995) Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374:269–271. doi:10.1038/374269a0
Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J (2000) Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of beta-hematin formation, and antiplasmodial activity. J Med Chem 43:283–291. doi:10.1021/jm990437l
Egan TJ, Mavuso WW, Ross DC, Marques HM (1997) Thermodynamic factors controlling the interaction of quinoline antimalarial drugs with ferriprotoporphyrin IX. J Inorg Biochem 68:137–145
Ekoue-Kovi K, Yearick K, Iwaniuk DP, Natarajan JK et al (2009) Synthesis and antimalarial activity of new 4-amino-7-chloroquinolyl amides, sulfonamides, ureas and thioureas. Bioorg Med Chem 17:270–283. doi:10.1016/j.bmc.2008.11.009
GraphPad Prism v3.0 (1999) GraphPad Software Inc., 10855 Sorrento Valley Rd. #203, San Diego. CA 92121
Hocart SJ, Liu H, Deng H, De D, Krogstad FM, Krogstad DJ (2011) 4-aminoquinolines active against chloroquine-resistant Plasmodium falciparum: basis of antiparasite activity and quantitative structure-activity relationship analyses. Antimicrob Agents Chemother 55:2233–2244. doi:10.1128/AAC.00675-10
Kaur K, Jain M, Reddy RP, Jain R (2010) Quinolines and structurally related heterocycles as antimalarials. Eur J Med Chem 45:3245–3264. doi:10.1016/j.ejmech.2010.04.011
Madrid PB, Wilson NT, DeRisi JL, Guy RK (2004) Parallel synthesis and antimalarial screening of a 4-aminoquinoline library. J Comb Chem 6:437–442. doi:10.1021/cc0340473
Malkov AV, Vrankova K, Cerny M, Kocovsky P (2009) On the selective N-methylation of BOC-protected amino acids The. J Org Chem 74:8425–8427. doi:10.1021/jo9016293
Na-Bangchang K, Karbwang J (2009) Current status of malaria chemotherapy and the role of pharmacology in antimalarial drug research and development. Fund Clin Pharmacol 23:387–409. doi:10.1111/j.1472-8206.2009.00709.x
Pandey AV, Bisht H, Babbarwal VK, Srivastava J, Pandey KC, Chauhan VS (2001) Mechanism of malarial haem detoxification inhibition by chloroquine. Biochem J 355:333–338
Prasad ASB, Kanth JVB, Periasamy M (1992) Convenient method for the reduction of amides, nitriles, carboxylic esters, acids and hydroboration of alkenes. Tetrahedron 48:4623–4628
Ray S, Madrid PB, Catz P, Levalley SE, Furniss MJ et al (2010) Development of a new generation of 4-aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models. J Med Chem 53:3685–3695. doi:10.1021/jm100057h
Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A et al (1996) 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother 40:1846–1854
Schlitzer M (2008) Antimalarial drugs—what is in use and what is in the pipeline. Arch Pharm 341:149–163. doi:10.1002/ardp.200700184
Sheehan JC, Hess GP (1955) A new method of forming peptide bonds. J Am Chem Soc 77:1067–1068. doi:10.1021/ja01609a099
Singh S, Srivastava RK, Srivastava M, Puri SK, Srivastava K (2011) In-vitro culture of Plasmodium falciparum: utility of modified (RPNI) medium for drug-sensitivity studies using SYBR Green I assay. Exp Parasitol 127:318–321. doi:10.1016/j.exppara.2010.08.007
Sinha M, Dola VR, Agarwal P, Srivastava K, Haq W, Puri SK, Katti SB (2014) Antiplasmodial activity of new 4-aminoquinoline derivatives against chloroquine resistant strain. Bioorg Med Chem 22:3573–3586. doi:10.1016/j.bmc.2014.05.024
Solomon VR, Haq W, Smilkstein M, Srivastava K, Puri SK, Katti SB (2010) 4-Aminoquinoline derived antimalarials: synthesis, antiplasmodial activity and heme polymerization inhibition studies. Eur J Med Chem 45:4990–4996. doi:10.1016/j.ejmech.2010.07.068
Solomon VR, Haq W, Smilkstein M, Srivastava K, Rajakumar S, Puri SK, Katti SB (2008) Synthesis and antimalarial activity of novel side chain modified antimalarial agents derived from 4-aminoquinoline. Med Chem 4:446–456. doi:10.2174/157340608785700207
Solomon VR, Haq W, Srivastava K, Puri SK, Katti SB (2007) Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J Med Chem 50:394–398. doi:10.1021/jm061002i
Solomon VR, Haq W, Srivastava K, Puri SK, Katti SB (2013) Design, synthesis of 4-aminoquinoline-derived thiazolidines and their antimalarial activity and heme polymerization inhibition studies. J Enzyme Inhib Med Chem 28:619–626. doi:10.3109/14756366.2012.666537
Solomon VR, Puri SK, Srivastava K, Katti SB (2005) Design and synthesis of new antimalarial agents from 4-aminoquinoline. Bioorg Med Chem 13:2157–2165. doi:10.1016/j.bmc.2004.12.051
Srivastava K, Puri S (2009) Recent Developments in Plasmodium falciparum in vitro model for Drug Discovery. Proc Natl Acad Sci, India 79:37–47
Stocks PA, Raynes KJ, Bray PG, Park BK, O’Neill PM, Ward SA (2002) Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J Med Chem 45:4975–4983. doi:10.1021/jm0108707
Sunduru N, Sharma M, Srivastava K, Rajakumar S, Puri SK, Saxena JK, Chauhan PM (2009) Synthesis of oxalamide and triazine derivatives as a novel class of hybrid 4-aminoquinoline with potent antiplasmodial activity. Bioorg Med Chem 17:6451–6462. doi:10.1016/j.bmc.2009.05.075
Teixeira C, Vale N, Perez B, Gomes A, Gomes JR, Gomes P (2014) “Recycling” classical drugs for Malaria. Chem Rev 114(22):11164–11220. doi:10.1021/cr500123g
Tripathi AK, Khan SI, Walker LA, Tekwani BL (2004) Spectrophotometric determination of de novo hemozoin/beta-hematin formation in an in vitro assay. Anal Biochem 325:85–91. doi:10.1016/j.ab.2003.10.016
Vasanth RD, Srinivasarao K, Katti SB (2014) Recent developments in the side chain modified 4-aminoquinolines as antimalarial agents. Chem Biol Interface 4:206–222
Wellems TE, Plowe CV (2001) Chloroquine-resistant malaria. J Infect Dis 184:770–776. doi:10.1086/322858
World Malaria Report (WHO) (2014) http://www.who.int/malaria/publications/world_malaria_report_2014/en/
Acknowledgments
One of the authors (K.S.R) thanks the CSIR, New Delhi, for Senior Research Fellowship. Authors thank the Director, CDRI, for the support, and the SAIF division for the spectral data. The CDRI Communication No is 9197.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Srinivasarao, K., Agarwal, P., Srivastava, K. et al. Design, synthesis, and in vitro antiplasmodial activity of 4-aminoquinolines containing modified amino acid conjugates. Med Chem Res 25, 1148–1162 (2016). https://doi.org/10.1007/s00044-016-1555-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1555-5