Abstract
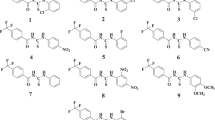
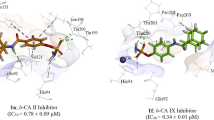
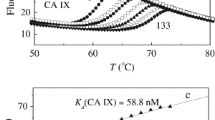
Benzamide sulfonamide and its derivatives were identified as potent inhibitors of carbonic anhydrase-II (bovine carbonic anhydrase-II (bCA-II) and human recombinant carbonic anhydrase-II (hCA-II)) with IC50 values 0.06–0.28 μM and 0.09–0.58 μM, respectively. Different kinetics parameter, such as V max, K m , and K i , were determined. Molecular docking simulation studies on the lead compounds were carried out by AutoDock Vina docking software. The compounds were also evaluated for their cytotoxicity against 3T3 (Normal Cell Lines Mouse Fibroblast) cell line. All the compounds have shown non-cytotoxic effect toward 3T3 cell lines. This study has identified novel scaffolds for further hit-to-lead optimization for the discovery of effective drugs against carbonic anhydrase-II-associated disorder, such as glaucoma, leukemia, cystic fibrosis, and epilepsy.






Similar content being viewed by others
References
Alterio V, Vitale RM, Monti SM, Pedone C, Scozzafava A, Cecchi A, De Simone G, Supuran CT (2006) Carbonic anhydrase inhibitors: X-ray and molecular modeling study for the interaction of a fluorescent antitumor sulfonamide with isozyme II and IX. J Am Chem Soc 128:8329–8335
Arslan O (2001) Inhibition of bovine carbonic anhydrase by new sulfonamide compounds. Biochemistry (Moscow) 66:982–983
Čapkauskaite E, Zubrienė A, Baranauskien L, Tamulaitiene G, Manakova E, Kairys V, Gražulis S, Tumkevičius S, Matulis D (2012) Design of [(2-pyrimidinylthio) acetyl] benzenesulfonamides as inhibitors of human carbonic anhydrases. Eur J Med Chem 51:259–270
Dimas K, Demetzos C, Marsellos M, Sotiriadou R, Malamas M, Kokkinopoulos D (1998) Cytotoxic activity of labdane type diterpenes against human leukemic cell lines in vitro. Planta Med 64:208–211
Ghiasi M, Kamalinahad S, Arabieh M, Zahedi M (2012) Carbonic anhydrase inhibitors: a quantum mechanical study of interaction between some antiepileptic drugs with active center of carbonic anhydrase enzyme. Comput Theor Chem 992:59–69
Gilmour KM (2010) Perspectives on carbonic anhydrase. Comp Biochem Physiol A Mol Integr Physiol 157:193–197
Huey R, Morris GM, Olson AJ, Goodsell DS (2007) A semiempirical free energy force field with charge-based desolvation. J Comput Chem 28:1145–1152
Köhlerr K, Hillebrecht A, Schulze Wischeler J, Innocenti A, Heine A, Supuran CT, Klebe G (2007) Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem 46:7697–7699
Leatherbarrow RJ (2010) GraFit version 7.00. E.S.L., Staines
Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT (2005a) Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 48:7860–7866
Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT (2005b) Carbonic anhydrase inhibitors: inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg Med Chem Lett 15:3828–3833
Nishimori I, Minakuchi T, Onishi S, Vullo D, Cecchi A, Scozzafava A, Supuran CT (2007) Carbonic anhydrase inhibitors: cloning, characterization, and inhibition studies of the cytosolic isozyme III with sulfonamides. Bioorg Med Chem 15:7229–7236
Saeed A, Zaib S, Pervez A, Mumtaz A, Shahid M, Iqbal J (2013) Synthesis, molecular docking studies, and in vitro screening of sulfanilamide–thiourea hybrids as antimicrobial and urease inhibitors. Med Chem Res 22:3653–3662
Saito R, Sato T, Ikai A, Tanaka N (2004) Structure of bovine carbonic anhydrase II at 1.95 A resolution. Acta Crystallogr D Biol Crystallogr 60:792–795
Shank RP, Doose DR, Streeter AJ, Bialer M (2005) Plasma and whole blood pharmacokinetics of topiramate: the role of carbonic anhydrase. Epilepsy Res 63:103–112
Smith KS, Ferry JG (2000) Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 24:335–366
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Supuran CT, Scozzafava A (2001) Carbonic anhydrase inhibitors. Curr Med Chem Immunol Endocr Metab Agents 1:61–97
Supuran CT, Ilies MA, Scozzafava A (1998) Carbonic anhydrase inhibitors-Part 29 1: interaction of isozymes I, II and IV with benzolamide-like derivatives. Eur J Med Chem 33:739–751
Temperini C, Innocenti A, Scozzafava A, Supuran CT (2008) Carbonic anhydrase activators: kinetic and X-ray crystallographic study for the interaction of d-and l-tryptophan with the mammalian isoforms I-XIV. Bioorg Med Chem 16:8373–8378
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT (2003) Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 13:1005–1009
Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT (2004) Carbonic anhydrase inhibitors. Inhibition of mitochondrial isozyme V with aromatic and heterocyclic sulfonamides. J Med Chem 47:1272–1279
Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT (2005a) Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides—a new target for the design of antitumor and antiglaucoma drugs? Bioorg Med Chem Lett 15:963–969
Vullo D, Voipio J, Innocenti A, Rivera C, Ranki H, Scozzafava A, Kaila K, Supuran CT (2005b) Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. J Med Chem 15:971–976
Acknowledgment
The authors gratefully acknowledge the HEC (Higher Education Commission of Pakistan), for financial support.
Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Saleem, M., Saeed, A., Atia-tul-Wahab et al. Benzamide sulfonamide derivatives: potent inhibitors of carbonic anhydrase-II. Med Chem Res 25, 438–448 (2016). https://doi.org/10.1007/s00044-015-1493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1493-7