Abstract
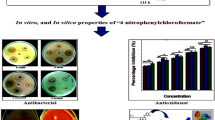
In search of new biologically active compounds, some novel (E)-1-aryl-2-(3,5-dimethyl-4-(aryldiazenyl)-1H-pyrazol-1-yl)ethanones 3a–u have been synthesized via solvent-free greener approach. All compounds were characterized on the basis of a combined use of IR, NMR (1H, 13C), mass spectrometry and elemental analyses. In order to establish the position of methyl groups in the compound 3b, 2D NMR (COSY, HSQC and HMBC) spectroscopic technique has also been used. To explore the biological potential, compounds were evaluated for their antimicrobial, antioxidant and UV-mediated DNA damage protective activity. Among the series, six compounds 3b, 3i, 3k, 3l, 3m and 3t were found to exhibit a very good level of antibacterial activity and four compounds 3g, 3o, 3p and 3t were emerged as an efficient class of antifungal agents in reference to the standard drugs, viz ciprofloxacin and amphotericin B, respectively. In studying the UV-mediated DNA damage protective activity, all compounds 3a–u (except 3d) protected the plasmid DNA from the UV damaging effect in which 3e was found to be the most potent DNA-protecting agent which avoids the transformation of the native supercoiled form into an open circular and linear form. In addition, the compounds 3f, 3m and 3t were found to be active in antioxidant screening using DPPH assay.







Similar content being viewed by others
References
Abdel-Aziz M, Abuo-Rahma GEDA, Hassan AA (2009) Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem 44:3480–3487
Bekhit AA, Ashour HM, Bekhit AD, Bekhit SA (2009) Synthesis and biological evaluation of novel pyrazole derivatives as anti-inflammatory antimicrobial agents. Med Chem 5:103–117
Bondet V, Brand-Williams W, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm Wiss Technol 30:609–615
Bondock S, Naser T, Ammar YA (2013) Synthesis of some new 2-(3-pyridyl)-4,5-disubstituted thiazoles as potent antimicrobial agents. Eur J Med Chem 62:270–279
Cai CX, Birk DE, Linsenmayer TF (1998) Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol Biol Cell 9:1037–1051
Deryabin D, Davydova O, Gryazeva I (2012) Alkylresorcinols protect the DNA from UV-damage in vitro and in vivo models. In: Verbeek CJ (ed) Products and applications of biopolymers. In Tech, Rijeka, pp 185–200
El-Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque C, Andrei G, Snoeck R, Balzarini J, Rashad AA (2009) Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem 44:3746–3753
Javed MASA, Hassan MH (2013) Synthesis and antimicrobial activity of pyrazoline and pyrazole analogues containing quinoline moiety. Indian J Chem, Sect B 52:1493–1499
Kalita S, Kumar G, Karthik L, Rao KVB (2012) In vitro antioxidant and DNA damage inhibition activity of aqueous extract of Lantana camara L. (Verbenaceae) leaves. Asian Pac J Trop Biomed 2:S1675–S1679
Kamal R, Kumar V, Bhardwaj V, Kumar V, Aneja KR (2015) Synthesis, characterization and in vitro antimicrobial evaluation of some novel hydrazone derivatives bearing pyrimidinyl and pyrazolyl moieties as a promising heterocycles. Med Chem Res 24:2551–2560
Kaur K, Kumar V, Sharma AK, Gupta GK (2014) Isoxazoline containing natural products as anticancer agents: a review. Eur J Med Chem 77:121–133
Krystof V, Cankar P, Frysova I, Slouka J, Kontopidis G, Dzubak P, Hajduch M, Srovnal J, de Azevedo Jr WF, Orsag XM, Paprskarova M, Rolcik J, Latr A, Fischer PM, Strnad M (2006) 4-Arylazo-3,5-diamino-1H-pyrazole CDK Inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effects. J Med Chem 49:6500–6509
Kumar V, Aggarwal R, Singh SP (2006) The reaction of hydroxylamine with aryl trifluoromethyl-β-diketones: synthesis of 5-hydroxy-5-trifluoromethyl-∆2-isoxazolines and their dehydration to 5-trifluoromethylisoxazoles. J Fluor Chem 127:880–888
Kumar V, Kaur K, Gupta GK, Kumar S (2013a) Developments in synthesis of the anti-inflammatory drug celecoxib: a review. Recent Pat Inflamm Allergy Drug Discov 7:124–134
Kumar V, Kaur K, Gupta GK, Sharma AK (2013b) Pyrazole containing natural products: synthetic preview and biological significance. Eur J Med Chem 69:735–753
Kumar V, Kaur K, Gupta GK (2014a) Triazole and oxadiazole containing natural products: a review. Nat Prod J 4:115–130
Kumar V, Kaur K, Karelia DN, Beniwal V, Gupta GK, Sharma AK, Gupta AK (2014b) Synthesis and biological evaluation of some 2-(3,5-dimethyl-1H-pyrazol-1-yl)-1-arylethanones: antibacterial, DNA photocleavage, and anticancer activities. Eur J Med Chem 81:267–276
Langley WD (1929) p-Bromophenacyl bromide. Org Synth 1:20
Langley WD (1941) p-Bromophenacyl bromide. Org Synth 1:127
Lee B, Kang P, Lee KH, Cho J, Nam W, Lee WK, Hur NH (2013) Solid-state and solvent-free synthesis of azines, pyrazoles, and pyridazinones using solid hydrazine. Tetrahedron Lett 54:1384–1388
Manojkumar P, Ravi TK, Gopalakrishan S (2009a) Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur J Med Chem 44:4690–4694
Manojkumar P, Ravi TK, Gopalakrishan S (2009b) Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm 59:159–170
Mert S, Kasmogullar R, Ica T, Colak F, Altun A, Ok S (2014) Synthesis, structure-activity relationships, and in vitro antibacterial and antifungal activity evaluations of novel pyrazole carboxylic and dicarboxylic acid derivatives. Eur J Med Chem 78:86–96
Oru EE, Koyigit-Kaymakioglu B, Oral B, Altunbas-Toklu HZ, Kabasakal L, Rollas S (2006) Synthesis of some novel azo derivatives of 3,5-dimethyl-1-(2-hydroxyethyl)pyrazole as potent analgesic agents. Arch Pharm Chem Life Sci 339:267–272
Raimondi MV, Maggio B, Raffa D, Plescia F, Cascioferro S, Cancemi G, Schillaci D, Cusimano MG, Vitale M, Daidone G (2012) Synthesis and anti-staphylococcal activity of new 4-diazopyrazole derivatives. Eur J Med Chem 58:64–71
Sankpal SA, Deshmukh MB, Anbhule PV, Salunkhe DK, Alsundkar KN, Patil PP, Chandam DR, Jagadale SD, Mulik AG, Rokade SS (2010) Synthesis and antibacterial evaluations of (3,5-dimethyl-1H-pyrazol-4-yl)-phenyl-diazenes. J Chem Pharm Res 2:574–579
Shehry MFE, Swellem RH, Abu-Bakr SM, El-Telbani EM (2010) Synthesis and molluscicidal evaluation of some new pyrazole, isoxazole, pyridine, pyrimidine, 1,4-thiazine and 1,3,4-thiadiazine derivatives incorporating benzofuran moiety. Eur J Med Chem 45:4783–4787
Shi JB, Tang WJ, Qi XB, Li R, Liu XH (2015) Novel pyrazole-5-carboxamide and pyrazole-pyrimidine derivatives: synthesis and anticancer activity. Eur J Med Chem 90:889–896
Shukla M, Seth DS, Kulshreshtha H (2013) Green chemical approach to synthesize 1-(N-substituted aniline malonyl)-3,5-dimethyl-4-(3,4-difluoro phenyl azo) pyrazoles and their antimicrobial evaluation. JOAC 2:1484–1488
Sojitra NA, Dixit RB, Patel RK, Patel JP, Dixit BC (2012) Classical and microwave assisted synthesis of new 4-(3,5-dimethyl-1-phenyl-1H-pyrazol-4-ylazo)-N-(2-substituted-4-oxo-4H-quinazolin-3-yl)benzenesulfonamide derivatives and their antimicrobial activities. J Saudi Chem Soc. doi:10.1016/j.jscs.2012.07.020
Taj T, Kamble RR, Gireesh TM, Hunnur RK, Margankop SB (2011) One-pot synthesis of pyrazoline derivatised carbazoles as antitubercular, anticancer agents, their DNA cleavage and antioxidant activities. Eur J Med Chem 46:4366–4373
Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62:410–415
Acknowledgments
Authors are thankful to Professor Dionisia Sanz and Professor Rosa M. Claramunt for providing a great help in elucidating the structure of the synthesized compounds. Authors are also thankful to Department of Science and Technology (DST), New Delhi, for awarding Senior Research Fellowship (SRF) under INSPIRE-DST scheme to Ms. Kamalneet Kaur. Authors pay sincere thanks to Maharishi Markandeshwar University, Mullana, for providing laboratory facilities and Mr. Avtar Singh, SAIF, Panjab University, Chandigarh, for providing NMR facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, K., Kumar, V., Beniwal, V. et al. Novel (E)-1-aryl-2-(3,5-dimethyl-4-(aryldiazenyl)-1H-pyrazol-1-yl)ethanones: solvent-free synthesis and antimicrobial, antioxidant and UV-mediated DNA damage protective activity studies. Med Chem Res 24, 4023–4036 (2015). https://doi.org/10.1007/s00044-015-1452-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1452-3