Abstract
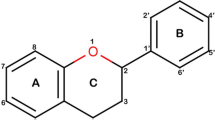
Limited oral bioavailability has hindered the widespread use of flavonoids as bioactive substances. Several studies have been performed to evaluate the transport characteristics of flavonoids in the intestines using cell models, such as Caco-2 cells, but information regarding the key structural features of flavonoids that influence intestinal uptake is still limited to date. In this study, quantitative structure–permeability relationship (QSPR) models were developed to study the permeability of 36 flavonoids through Caco-2 cells using both 2D and 3D approaches. For the 2D model, stepwise multiple linear regression (SMLR) and partial least squares regression (PLSR) resulted in good internal (R 2SMLR = 0.8, R 2PLSR = 0.93) and external (R 2SMLR = 0.93, R 2PLSR = 0.90) predictability using a set of 409 molecular descriptors. The high cross-validated (leave-one-out) R 2 values (Q 2) for both 2D models (Q 2SMLR = 0.77, Q 2PLSR = 0.67) suggest that the models are robust and predictive. A pharmacophore (GALAHAD)-based COMSIA analysis was used to generate the 3D QSPR model, which yielded a predictive and robust model (R 2training = 0.96, R 2test = 0.95, Q 2 = 0.625) composed of hydrogen bond acceptor and donor fields. According to the contour plots, the locations of hydrogen bond acceptors and donors play a crucial role in determining Caco-2 permeability of flavonoids. The models provide deeper insight into the QSPR of flavonoids on intestinal absorption using Caco-2 cell models and could be useful for the high-throughput screening of flavonoids and/or flavonoid-like drugs with health-promoting activities.
Graphical Abstract







Similar content being viewed by others
References
Actis-Goretta L, Ottaviani JI, Fraga CG (2005) Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem 54:229–234. doi:10.1021/jf052263o
Al Shukor N, Van Camp J, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, Raes K, Smagghe G (2013) Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J Agric Food Chem 61:11832–11839. doi:10.1021/jf404641v
Balasuriya N, Rupasinghe HPV (2012) Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem 135:2320–2325. doi:10.1016/j.foodchem.2012.07.023
Mevik B, Wehrens R, Liland K (2013) pls: Partial Least Squares and Principal Component regression. R package version 2.4–3. http://mevik.net/work/software/pls.html
Brand W, Shao J, Hoek-van den Hil EF, van Elk KN, Spenkelink B, de Haan LH, Rein MJ, Dionisi F, Williamson G, van Bladeren PJ, Rietjens IM (2010) Stereoselective conjugation transport and bioactivity of s- and R-hesperetin enantiomers in vitro. J Agric Food Chem 58:6119–6125. doi:10.1021/jf1008617
Bravi G, Gancia E, Mascagni P, Pegna M, Todeschini R, Zaliani A (1997) MS-WHIM, new 3D theoretical descriptors derived from molecular surface properties: a comparative 3D QSAR study in a series of steroids. J Comput Aided Mol Des 11:79–92
Bush B, Nachbar R Jr (1993) Sample-distance partial least squares: PLS optimized for many variables, with application to CoMFA. J Comput-Aided Mol Des 7:587–619. doi:10.1007/BF00124364
Butina D (2004) Performance of Kier-Hall E-state descriptors in quantitative structure activity relationship (QSAR) studies of multifunctional molecules. Molecules 9:1004–1009
Chen Y, Li H, Tang W, Zhu C, Jiang Y, Zou J, Yu Q, You Q (2009) 3D-QSAR studies of HDACs inhibitors using pharmacophore-based alignment European. J Med Chem 44:2868–2876. doi:10.1016/j.ejmech.2008.12.008
Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJC, Morgan MRA, Williamson G (1998) Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett 436:71–75. doi:10.1016/S0014-5793(98)01101-6
Egan WJ, Lauri G (2002) Prediction of intestinal permeability. Adv Drug Deliv Rev 54:273–289. doi:10.1016/S0169-409X(02)00004-2
Egan WJ, Merz KM, Baldwin JJ (2000) Prediction of drug absorption using multivariate statistics. J Med Chem 43:3867–3877. doi:10.1021/jm000292e
Farrell TL, Poquet L, Dew TP, Barber S, Williamson G (2012) Predicting phenolic acid absorption in Caco-2 cells: a theoretical permeability model and mechanistic study. Drug Metab Dispos 40:397–406. doi:10.1124/dmd.111.041665
Fiol M, Adermann S, Neugart S, Rohn S, Mugge C, Schreiner M, Krumbein A, Kroh L (2012) Highly glycosylated and acylated flavonols isolated from kale (Brassica oleracea var. sabellica)—Structure–antioxidant activity relationship. Food Res Int 47:80–89. doi:10.1016/j.foodres.2012.01.014
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276. doi:10.1016/S1093-3263(01)00123-1
Gonzales GB, Raes K, Coelus S, Struijs K, Smagghe G, Van Camp J (2014) Ultra(high)-pressure liquid chromatography-electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J Chromatogr A 1323:39–48. doi:10.1016/j.chroma.2013.10.077
Gramatica P (2006) WHIM descriptors of shape. QSAR Comb Sci 25:327–332. doi:10.1002/qsar.200510159
Hall LH, Mohney B, Kier LB (1991) The electrotopological state: structure information at the atomic level for molecular graphs. J Chem Inf Comput Sci 31:76–82. doi:10.1021/ci00001a012
Hashimoto K, Shimizu M (1993) Epithelial properties of human intestinal Caco-2 cells cultured in a serum-free medium. Cytotechnology 13:175–184
Hayeshi R, Hilgendorf C, Artursson P, Augustijns P, Brodin B, Dehertogh P, Fisher K, Fossati L, Hovenkamp E, Korjamo T, Masungi C, Maubon N, Mols R, Müllertz A, Mönkkönen J, O'Driscoll C, Oppers-Tiemissen HM, Ragnarsson EG, Rooseboom M, Ungell AL (2008) Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur J Pharm Sci 35:383–396. doi:10.1016/j.ejps.2008.08.004
Hsu CL, Yen GC (2008) Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res 52:53–61. doi:10.1002/mnfr.200700393
Huuskonen JJ, Villa AE, Tetko IV (1999) Prediction of partition coefficient based on atom-type electrotopological state indices. J Pharm Sci 88:229–233. doi:10.1021/js980266s
Huuskonen J, Rantanen J, Livingstone D (2000) Prediction of aqueous solubility for a diverse set of organic compounds based on atom-type electrotopological state indices. Eur J Med Chem 35:1081–1088. doi:10.1016/S0223-5234(00)01186-7
Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M (2000) Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130:1695–1699
Jing P, Zhao S, Ruan S, Sui Z, Chen L, Jiang L, Qian B (2014) Quantitative studies on structure–ORAC relationships of anthocyanins from eggplant and radish using 3D-QSAR. Food Chem 145:365–371. doi:10.1016/j.foodchem.2013.08.082
Kier L, Hall L (1990) An electrotopological-state index for atoms in molecules. Pharm Res 7:801–807. doi:10.1023/A:1015952613760
Lévèques A, Actis-Goretta L, Rein MJ, Williamson G, Dionisi F, Giuffrida F (2012) UPLC–MS/MS quantification of total hesperetin and hesperetin enantiomers in biological matrices. J Pharm Biomed Anal 57:1–6. doi:10.1016/j.jpba.2011.08.031
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26. doi:10.1016/S0169-409X(00)00129-0
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells:implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J (2002) Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr 132:1956–1961
Németh K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA (2003) Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 42:29–42. doi:10.1007/s00394-003-0397-3
Ollila F, Halling K, Vuorela P, Vuorela H, Slotte JP (2002) Characterization of flavonoid-biomembrane interactions. Arch Biochem Biophys 399:103–108. doi:10.1006/abbi.2001.2759
Olsen H, Aaby K, Borge GIA (2010) Characterization, quantification, and yearly variation of the naturally occurring polyphenols in a common red variety of curly kale (Brassica oleracea L. convar. acephala var. sabellica cv. ‘Redbor’). J Agric Food Chem 58:11346–11354. doi:10.1021/jf102131g
Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL (2011) The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med 50:237–244. doi:10.1016/j.freeradbiomed.2010.11.005
Pietta P-G (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. doi:10.1021/np9904509
Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M (2013) Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 75:588–602. doi:10.1111/j.1365-2125.2012.04425.x
Rose K, Hall LH, Kier LB (2002) Modeling blood-brain barrier partitioning using the electrotopological state. J Chem Inf Comput Sci 42:651–666
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Shimizu M (2010) Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem 74:232–241
Tammela P, Laitinen L, Galkin A, Wennberg T, Heczko R, Vuorela H, Slotte JP, Vuorela P (2004) Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch Biochem Biophys 425:193–199. doi:10.1016/j.abb.2004.03.023
Thilakarathna S, Rupasinghe H (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5:3367–3387
Tian XJ, Yang XW, Yang X, Wang K (2009) Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int J Pharm 367:58–64. doi:10.1016/j.ijpharm.2008.09.023
van de Waterbeemd H, Gifford E (2003) ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2:192–204
Wang Y, Wu Q, Yang X-W, Yang X, Wang K (2011) The membrane transport of flavonoids from Crossostephium chinense across the Caco-2 monolayer. Biopharm Drug Dispos 32:16–24. doi:10.1002/bdd.735
Wen X, Walle T (2006) Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos 34:1786–1792. doi:10.1124/dmd.106.011122
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst 58:109–130. doi:10.1016/S0169-7439(01)00155-1
Xie M, Zhang X (2013) MLRMPA: A package for Multilinear Regression Model Population Analysis. R package version 1.0. http://cran.r-project.org/web/packages/MLRMPA/index.html
Yap CW (2011) PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32:1466–1474. doi:10.1002/jcc.21707
Acknowledgments
The authors would like to thank the Special Research Fund (BOF) of the Ghent University for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzales, G.B., Van Camp, J., Zotti, M. et al. Two- and three-dimensional quantitative structure–permeability relationship of flavonoids in Caco-2 cells using stepwise multiple linear regression (SMLR), partial least squares regression (PLSR), and pharmacophore (GALAHAD)-based comparative molecular similarity index analysis (COMSIA). Med Chem Res 24, 1696–1706 (2015). https://doi.org/10.1007/s00044-014-1241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1241-4