Abstract
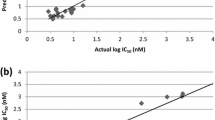
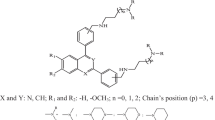
We report combined study of k-nearest neighbor, pharmacophore and 2D QSAR was performed on a series of 2,4-diaminopyrimidines dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum as antimalarial agents to gain insights into the structural determinants and their structure–activity relationship. The QSAR models for the prediction of activity of antiplasmodial activities against P. falciparum clones with wild-type TM4/8.2 and K1CB1 strains have been developed by the SA-PLS and SW-PLS methods, and the proposed models gain satisfactory results. The statistically significant best 2D QSAR model having correlation coefficient r 2 = 0.8569, 0.7853 and cross-validated squared correlation coefficient q 2 = 0.7104, 0.7216 with external predictive ability of pred_r 2 = 0.7995, 0.7064 was developed by wild-type TM4/8.2 and K1CB1 strains with SA-PLS. 3D QSAR studies using k-nearest neighbor molecular field analysis (kNN-MFA) method, identifies two models obtained by SA-PLS and SW-PLS methods leading to antimalarial activity prediction. The obtained pharmacophore model with lowest RMSD value (0.1548 Å), consisting of one hydrogen donor, two hydrogen acceptors and one aromatic region was developed. These models were found to yield reliable clues for further optimization of 2,4-diaminopyrimidines derivatives in the data set. We hope that these results will give new insights into chemical modifications that can be realized with the aim of designing new inhibitors with improved pharmacological properties.



Similar content being viewed by others
References
Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N (2004) Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363(9402):9–17
Ajmani S, Jhadav K, Kulkarni SA (2006) Three-dimensional QSAR using the k-nearest neighbor method and its interpretation. Chem Inf Mod 46:24–31
Baumann K (2000) An alignment-independent versatile structure descriptor for QSAR and QSPR based on the distribution of molecular features. J Chem Inf Comput Sci 42:26–35
Blakley RL (1984) Dihydrofolate reductase. In: Blakley RL, Benkovic SJ (eds) Folates and pteridines, vol 1. Wiley, New York, pp 191–253
Cowman AF, Morry MJ, Biggs BA, Cross GAM, Foote SJ (1988) Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci USA 85:9109–9113
Cramer RD III, Bunce JD, Patterson DE (1998) Cross validation, bootstrapping, and partial least squares compared with multiple regression in conventional QSAR studies. Quant Struct Act Relat 7:18–25
Darlington RB (1990) Regression and linear models. McGraw-Hill, New York
Dearden JC, Cronin MT, Kaiser KL (2009) How not to develop a quantitative structure-activity or structure–property relationship (QSAR/QSPR). SAR QSAR Environ Res 20:241–266
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron 36:3219–3228
Ghosh P, Bagchi MC (2009) QSAR modeling for quinoxaline derivatives using genetic algorithm and simulated annealing based feature selection. Curr Med Chem 16:4032–4048
Golbraikh A, Tropsha A (2002) Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. J Comput Aided Mol Des 16:357–369
Halgren TA (1996) Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J Comp Chem 17:520–552
Hou TJ, Wang JM, Xu XJ (1999) Applications of genetic algorithms on the structure–activity correlation study of a group of non-nucleoside HIV-1 inhibitors. Chem Intell Lab Syst 45:303–310
Liedl KR, Rode Bernd M, Sotriffer CA, Varga JM, Winger RH (1996) Comparative docking studies on ligand binding to the specific antibodies IgE-La2 and IgE-Lb4. J Comput Aided Mol Des 10:305–320
Matthew Cross R, Namelikonda NK, Mutka TS, Luong L, Kyle DE, Manetsch R (2011) Synthesis, antimalarial activity, and structure–activity relationship of 7-(2-phenoxyethoxy)-4(1H)-quinolones. J Med Chem 54:8321–8327
Molyneaux CA, Krugliak M, Ginsburg H, Chibale K (2005) Arylpiperazines displaying preferential potency against chloroquine-resistant strains of the malaria parasite Plasmodium falciparum. Biochem Pharmacol 71:61
Muraleedharan KM, Avery MA (2009) Progress in the development of peroxide-based anti-parasitic agents. Drug Discov Today 14:793
Murray MC, Perkins ME (1996) Chemotherapy of malaria. Ann Rep Med Chem 31:141–150
Peterson DS, Milhous WK, Wellems TE (1990) Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA 87:3018–3022
Rastelli G, Sirawaraporn W, Sompornpisut P, Vilaivan T, Kamchonwongpaisan S, Quarrell R, Lowe G, Thebtaranonth Y, Yuthavong Y (2000) Interaction of pyrimethamine, cycloguanil, WR99210 and their analogues with Plasmodium falciparum dihydrofolate reductase: structural basis of antifolate resistance. Bioorg Med Chem 8:1117–1128
Shen M, LeTiran A, Xiao Y, Golbraikh A, Kohn H, Tropsha A (2002) Quantitative structure-activity relationship analysis of functionalized amino acid anticonvulsant agents using k nearest neighbor and simulated annealing PLS methods. J Med Chem 4:2811–2823
Sirawaraporn W, Prapunwattana P, Sirawaraporn R, Yuthavong Y, Santi DV (1993) The dihydrofolate reductase domain of Plasmodium falciparum thymidylate synthase-dihydrofolate reductase: gene synthesis, expression, and anti-folate resistant mutants. J Biol Chem 268:21637–21644
Tarnchompoo B, Sirichaiwat C, Phupong W, Intaraudom C, Sirawaraporn W, Kamchonwongpaisan S, Vanichtanankul J, Thebtaranonth Y, Yuthavong Y (2002) Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum. J Med Chem 45:1244–1252
Tropsha A (2010) Best practices for QSAR model development, validation, and exploitation. Mol Inf 29:476–488
Vlife Sciences Technologies Pvt. Ltd (2008) VLife MDS 3.5 Molecular design suite. Vlife Sciences Technologies Pvt. Ltd., Pune
White NJ (1998) Why is it that antimalarial drug treatments do not always work? Ann Trop Med Parasitol 92:449–458
White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, Kokwaro G, Ouma J, Hien TT, Molyneux ME, Taylor TE, Newbold CI, Ruebush TK 2nd, Danis M, Greenwood BM, Anderson RM, Olliaro P (1999) Averting a malaria disaster. Lancet 353:1965–1967
Willcox ML, Bodeker G (2004) Traditional herbal medicines for malaria. BMJ 329:1156
WHO (1997) Weekly epidemiol. Report 72, 269
Zheng W, Tropsha A (2000) Novel variable selection quantitative structure–property relationship approach based on the k-nearest neighbor principle. J Chem Inf Comput Sci 40:185–194
Acknowledgments
The author wishes to express gratitude to V-life Science Technologies Pvt. Ltd for providing the software for the study.
Conflict of interest
The authors declare no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, M.C., Sharma, S. & Bhadoriya, K.S. Molecular modeling studies on substituted aminopyrimidines derivatives as potential antimalarial compounds. Med Chem Res 24, 1272–1288 (2015). https://doi.org/10.1007/s00044-014-1199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1199-2