Abstract
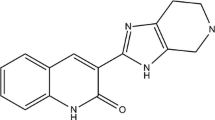
The present study is an attempt to formulate the three-dimensional quantitative structure–activity relationship (3D-QSARs) modeling of 3-benzimidazol-2-ylhydroquinolin-2-one derivatives inhibiting vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase. The 3D-QSARs were established for 36 3-benzimidazol-2-ylhydroquinolin-2-one derivatives as VEGFR-2 tyrosine kinase inhibitors using comparative molecular field analysis (CoMFA) and comparative similarity indices analysis (CoMSIA) techniques. The negative logarithm of IC50 (pIC50) was used as the biological activity in the 3D-QSAR study. With the CoMFA model, the cross-validated value (q 2) was 0.516, the non-cross-validated value (R 2) was 0.927, and the external cross-validated value (Q 2ext ) was 0.855; with the CoMSIA model, the corresponding q 2, R 2, and Q 2ext values were 0.538, 0.980, and 0.809, respectively. The CoMFA and CoMSIA models were validated by a structurally diversified test set of nine compounds. Then, molecular docking was carried out to better understand of the interactions between VEGFR-2 tyrosine kinase target and inhibitors. Finally, based on results of the structure–activity relationship and of the molecular docking, seven VEGFR-2 tyrosine kinase inhibitors that showed excellent potencies have been constructed.






Similar content being viewed by others
References
Bhargava P, Robinson MO (2011) Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep 13:103–111
Böhm M, Stürzebecher J, Klebe G (1999) Three-dimensional quantitative structure-activity relationship analyses using comparative molecular field analysis and comparative molecular similarity indices analysis to elucidate selectivity differences of inhibitors binding to trypsin, thrombin, and factor Xa. J Med Chem 42:458–477
Bush BL, Nachbar RB Jr (1993) Sample-distance partial least squares: PLS optimized for many variables, with application to CoMFA. J Comput Aided Mol Des 7:587–619
Cai Z, Wei D, Borzilleri RM, Qian L, Kamath A, Mortillo S, Wautlet B, Henley BJ, Jeyaseelan R Sr, Tokarski J, Hunt JT, Bhide RS, Fargnoli J, Lombardo LJ (2008) Synthesis, SAR, and Evaluation of 4-[2,4-Difluoro-5-(cyclopropylcarbamoyl)phenylamino]pyrrolo[2,1-f][1,2,4]triazine-based VEGFR-2 kinase inhibitors. Bioorg Med Chem Lett 18:1354–1358
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–398
Clark M, Cramer RD, Van Opdenbosch N (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10:982–1012
Cramer RD III (1993) Partial least squares (PLS): its strengths and limitations. Perspect Drug Discov Des 1:269–278
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110:5959–5967
Ding L, Tang F, Huang W, Jin Q, Shen H, Wei P (2013) Design, synthesis, and biological evaluation of novel 3-pyrrolocyclohexylene-2-dihydroindolinone derivatives as potent receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett 23:5630–5633
Doi T, Ohtsu A, Fuse N, Yoshino T, Tahara M, Shibayama K, Takubo T, Weinreich DM (2013) Phase 1 study of trebananib (AMG 386), an angiogenesis targeting angiopoietin-1/2 antagonist, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 71:227–235
Golbraikh A, Tropsha A (2002) Beware of q2. J Mol Graph Model 20:269–276
Gupta N, Vyas VK, Patel B, Ghate M (2014) Predictive 3D-QSAR and HQSAR model generation of isocitrate lyase (ICL) inhibitors by various alignment methods combined with docking study. Med Chem Res 23:2757–2768
Jeltsch M, Leppänen V, Saharinen P, Alitalo K (2013) Receptor Tyrosine Kinase-Mediated Angiogenesis. Cold Spring Harb Perspect Biol 5:a9183
Klebe G, Abraham U, Mietzner T (1994) Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37:4130–4146
Knowles PP, Murray-Rust J, Kjær S, Scott RP, Hanrahan S, Santoro M, Ibáñez CF, McDonald NQ (2006) Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem 281:33577–33587
Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng R, Chen Y, Emerson L, Langmuir P, Manegold C (2012) Vandetanib versus placebo in patients with advanced non–small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 30:1114–1121
McIntosh AR, Bookstein FL, Haxby JV, Grady CL (1996) Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3:143–157
Ramsden JD (2000) Angiogenesis in the thyroid gland. J Endocrinol 166:475–480
Renhowe PA, Pecchi S, Shafer CM, Machajewski TD, Jazan EM, Taylor C, Antonios-McCrea W, McBride CM, Frazier K, Wiesmann M (2009) Design, structure–activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: a novel class of receptor tyrosine kinase inhibitors. J Med Chem 52:278–292
Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19
Steeghs N, Gelderblom H, Wessels J, Eskens FA, de Bont N, Nortier JW, Guchelaar H (2011) Pharmacogenetics of telatinib, a VEGFR-2 and VEGFR-3 tyrosine kinase inhibitor, used in patients with solid tumors. Invest New Drugs 29:137–143
Viswanadhan VN, Ghose AK, Revankar GR, Robins RK (1989) Atomic physicochemical parameters for three-dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J Chem Inf Comput Sci 29:163–172
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 58:109–130
Xiao A, Zhang Z, An L, Xiang Y (2008) 3D-QSAR and docking studies of 3-arylquinazolinethione derivatives as selective estrogen receptor modulators. J Mol Model 14:149–159
Acknowledgments
The project was supported by the National Natural Science Foundation of China (21072111, 21172070, and 21272131) and the Shandong Provincial Natural Science Foundation, China (ZR2011BM015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, Cm., Liu, Dq., Wang, Xy. et al. 3D-QSAR and docking study on 3-benzimidazol-2-ylhydroquinolin-2-one derivatives as VEGFR-2 tyrosine kinase inhibitors. Med Chem Res 24, 934–943 (2015). https://doi.org/10.1007/s00044-014-1161-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1161-3