Abstract
Statistical association between congenital autism and prenatal exposure to ritodrine (4-(2-((1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-ylamino)ethyl)phenol) as a tocolytic agent was a matter of concern. Moreover, neuronal nitric oxide momentous role in various behavioral and cognitive functions was reported. In this context, a correlation between prenatal exposures to ritodrine, neuronal nitric oxide level and autism occurrence must be investigated. For this reason, we proposed possible inhibition of neuronal nitric oxide synthase (nNOS) by ritodrine. An insight toward our hypothesis approval was done through docking ritodrine into the catalytic pocket of nNOS. Apparently, ritodrine shared at least five critical binding interactions with a potent nNOS inhibitor (PDB code: JI7). Subsequent in vitro experiment pointed out that ritodrine indeed inhibited the enzymatic activity of nNOS at low micromolar level. As a conclusion, ritodrine should not be used as a tocolytic agent but as a novel non peptidomimetic nNOS inhibitor lead scaffold for future optimization.
Graphical Abstract
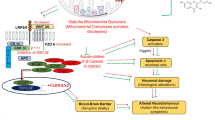
(A) Ritodrine chemical structure (B) Docked pose of ritodrine into nNOS-binding pocket (PDB code: 3B3P, resolution 2.6 Ǻ) (C) Docked pose of inhibitor JI7 (green) as produced by docking simulation and the crystallographic structure of this inhibitor within nNOS




Similar content being viewed by others
References
Abu Hammad AM, Afifi FU, Taha MO (2007) Combining docking, scoring and molecular field analyses to probe influenza neuraminidase-ligand interactions. J Mol Graph Model 26:443–456. doi:10.1016/j.jmgm.2007.02.002
Akbarian S, Bunney WE, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG (1993) Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 50:169–177
Berkman ND et al (2003) Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol 188:1648–1659. doi:10.1067/mob.2003.356
Bloom FE (1993) Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry 50:224–227
Bugajski J, Gadek-Michalska A, Bugajski A (2004) Nitric oxide and prostaglandin systems in the stimulation of hypothalamic-pituitary-adrenal axis by neurotransmitters and neurohormones. J Physiol Pharmacol 55:679–703
Bustanji Y, Taha MO, Yousef AM, Al-Bakri AG (2006) Berberine potently inhibits protein tyrosine phosphatase 1B: investigation by docking simulation and experimental validation. J Enzyme Inhib Med Chem 21:163–171. doi:10.1080/14756360500533026
Chiavegatto S, Nelson RJ (2003) Interaction of nitric oxide and serotonin in aggressive behavior. Horm Behav 44:233–241. doi:10.1016/j.yhbeh.2003.02.002
Dennedy MC, Friel AM, Gardeil F, Morrison JJ (2001) Beta-3 versus beta-2 adrenergic agonists and preterm labour: in vitro uterine relaxation effects. Br J Obstet Gynaecol 108:605–609. doi:10.1111/j.1471-0528.2001.00147.x
Derbent A, Simavli S, Gümüş İ, Tatli M, Turhan N (2011) Nifedipine for the treatment of preterm labor in twin and singleton pregnancies. Arch Gynecol Obstet 284:821–826. doi:10.1007/s00404-010-1751-3
Edwards TM, Rickard NS (2007) New perspectives on the mechanisms through which nitric oxide may affect learning and memory processes. Neurosci Biobehav Rev 31:413–425. doi:10.1016/j.neubiorev.2006.11.001
Fujimoto S, Akahane M, Sakai A (1986) Concentrations of ritodrine hydrochloride in maternal and fetal serum and amniotic fluid following intravenous administration in late pregnancy. Eur J Obstet Gynecol Reprod Biol 23:145–152
Fujimoto S, Tanaka T, Akahane M (1991) Levels of ritodrine hydrochloride in fetal blood and amniotic fluid following long-term continuous administration in late pregnancy. Eur J Obstet Gynecol Reprod Biol 38:15–18
Galecki P, Maes M, Florkowski A, Lewinski A, Galecka E, Bienkiewicz M, Szemraj J (2011) Association between inducible and neuronal nitric oxide synthase polymorphisms and recurrent depressive disorder. J Affect Disorders 129:175–182. doi:10.1016/j.jad.2010.09.005
Galimberti D et al (2005) Association of neuronal nitric oxide synthase C276T polymorphism with Alzheimer’s disease. J Neurol 252:985–986. doi:10.1007/s00415-005-0783-2
Ghigo D, Riganti C, Gazzano E, Costamagna C, Bosia A (2006) Cycling of NADPH by glucose 6-phosphate dehydrogenase optimizes the spectrophotometric assay of nitric oxide synthase activity in cell lysates. Nitric Oxide 15:148–153. doi:10.1016/j.niox.2006.01.002
Gomez-Vidal JA, Martasek P, Roman LJ, Silverman RB (2004) Potent and selective conformationally restricted neuronal nitric oxide synthase inhibitors. J Med Chem 47:703–710. doi:10.1021/jm030297m
Haddersalgra M, Touwen BCL, Huisjes HJ (1986) Long-term follow-up of children prenatally exposed to ritodrine. Br J Obstet Gynaecol 93:156–161. doi:10.1111/j.1471-0528.1986.tb07880.x
Hah JM, Silverman RB (2001) Reduced amide bond peptidomimetics. (4s)-N-(4-amino-5- aminoalkylaryl aminopentyl-N′-nitroguanidines, potent and selective inhibitors of neuronal nitric oxide synthase. Abstr Pap Am Chem S 222:U683
Hah JM, Martasek P, Roman LJ, Silverman RB (2003) Aromatic reduced amide bond peptidomimetics as selective inhibitors of neuronal nitric oxide synthase. J Med Chem 46:1661–1669. doi:10.1021/jm0202932
Hancock DB, Martin ER, Vance JM, Scott WK (2008) Nitric oxide synthase genes and their interactions with environmental factors in Parkinson’s disease. Neurogenetics 9:249–262. doi:10.1007/s10048-008-0137-1
Heneka MT, Feinstein DL (2001) Expression and function of inducible nitric oxide synthase in neurons. J Neuroimmunol 114:8–18. doi:10.1016/s0165-5728(01)00246-6
Huang H, Martasek P, Roman LJ, Masters BSS, Silverman RB (1999) N-omega-nitroarginine-containing dipeptide amides. Potent and highly selective inhibitors of neuronal nitric oxide synthase. J Med Chem 42:3147–3153. doi:10.1021/jm990111c
Igarashi J et al (2009) Crystal structures of constitutive nitric oxide synthases in complex with de novo designed inhibitors. J Med Chem 52:2060–2066. doi:10.1021/jm900007a
Ji H, Gomez-Vidal JA, Martasek P, Roman LJ, Silverman RB (2006) Conformationally restricted dipeptide amides as potent and selective neuronal nitric oxide synthase inhibitors. J Med Chem 49:6254–6263. doi:10.1021/jm0604124
Ji H et al (2008) Minimal pharmacophoric elements and fragment hopping, an approach directed at molecular diversity and isozyme selectivity. Design of selective neuronal nitric oxide synthase inhibitors. J Am Chem Soc 130:3900–3914. doi:10.1021/ja0772041
Kim H-W et al (2009) Family-based association study between NOS-I and -IIA polymorphisms and autism spectrum disorders in Korean trios. Am J Med Genet 150B:300–306. doi:10.1002/ajmg.b.30798
Lemmon G, Meiler J (2013) Towards ligand docking including explicit interface water molecules. PLoS ONE 8:e67536. doi:10.1371/journal.pone.0067536
Leveno KJ, Little BB, Cunningham FG (1990) The national impact of ritodrine hydrochloride for inhibition of preterm labor. Obstet Gynecol 76:12–15
Liu S, Fu R, Zhou LH, Chen SP (2012) Application of consensus scoring and principal component analysis for virtual screening against beta-secretase (BACE-1). PLoS ONE 7:e38086
Muegge I (2000) A knowledge-based scoring function for protein-ligand interactions: probing the reference state. Perspect Drug Discov 20:99–114. doi:10.1023/a:1008729005958
Patrick GL (2005) An introduction to medicinal chemistry, 3rd edn. Oxford University Press, New York
Schwarz MK, Page P (2003) Preterm labour: an overview of current and emerging therapeutics. Curr Med Chem 10:1441–1468. doi:10.2174/0929867033457331
Seo J, Igarashi J, Li H, Martasek P, Roman LJ, Poulos TL, Silverman RB (2007) Structure-based design and synthesis of N-omega-nitro-l-arginine-containing peptidomimetics as selective inhibitors of neuronal nitric oxide synthase. Displacement of the heme structural water. J Med Chem 50:2089–2099. doi:10.1021/jm061305c
Silverman RB (2009) Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases. Acc Chem Res 42:439–451. doi:10.1021/ar800201v
Suaifan GARY, Goodyer CLM, Threadgill MD (2010) Synthesis of N-(Methoxycarbonylthienylmethyl) thioureas and evaluation of their interaction with inducible and neuronal nitric oxide synthase. Molecules 15:3121–3134. doi:10.3390/molecules15053121
Suaifan GARY, Al-Ejal HAN, Taha MO (2012a) Pharmacophore and QSAR modeling of endothelial nitric oxide synthase inhibitors and subsequent validation and in silico search for new hits. JJPS 5:220–242
Suaifan GARY, Shehadehh M, Al-Ijel H, Taha MO (2012b) Extensive ligand-based modeling and in silico screening reveal nanomolar inducible nitric oxide synthase (iNOS) inhibitors. J Mol Graph Model 37:1–26. doi:10.1016/j.jmgm.2012.04.001
Van Lierde M, Thomas K (1982) Ritodrine concentrations in maternal and fetal serum and amniotic fluid. J Perinat Med 10:119–124
Volke V, Wegener G, Bourin M, Vasar E (2003) Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res 140:141–147. doi:10.1016/s0166-4328(02)00312-1
Witter FR, Zimmerman AW, Reichmann JP, Connors SL (2009) In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol 201:553–559. doi:10.1016/j.ajog.2009.07.010
Zhou L, Zhu D-Y (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20:223–230. doi:10.1016/j.niox.2009.03.001
Acknowledgments
The authors would like to acknowledge the Deanship of the Scientific Research, The University of Jordan (Grant number 1444) and Hamdi Mango Center for Scientific Research and Sandeep Khosla (King’s College London) for help with in vitro assays.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suaifan, G.A.R.Y., Shehadeh, M.B., Al-Ijel, H.A.N. et al. Ritodrine inhibits neuronal nitric oxide synthase, a potential link between tocolysis and autism. Med Chem Res 23, 5102–5109 (2014). https://doi.org/10.1007/s00044-014-1066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1066-1