Abstract
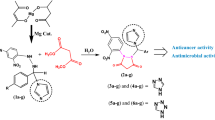
A series of new 2,5-disubstituted 1,3,4-oxadiazoles have been conveniently synthesized through an oxidative C–O coupling by direct C–H bond activation of N-aroyl-N-arylidinehydrazines using a catalytic quantity of CuO nanoparticles. Twenty compounds have been synthesized in good to excellent yields (75–90 %). All the synthesized compounds were evaluated for their in vitro antiproliferative, antibacterial, and antifungal activity. Compounds 8d and 10d are more promising antiproliferative agents with IC50 value of 3.66 and 3.89 µM in MCF-7 cell line, and compounds 8a and 10a were showed more potent antifungal activity than standard drug.







Similar content being viewed by others
References
Aboraia AS, Rahman HMA, Mahfouz NM, Gendy MAE (2006) Novel 5-(2-hydroxyphenyl)-3-substituted-2,3-dihydro-1,3,4-oxadiazole-2-thione derivatives: promising anticancer agents. Bioorg Med Chem 14:1236–1246
Al-Talib M, Tashtoush H, Odeh N (1990) A convenient synthesis of alkyl and aryl substituted bis-1,3,4-oxadiazoles. Synth Commun 20:1811–1817
Babu SG, Ramasamy K (2011) CuO nanoparticles: a simple, effective, ligand free and reusable heterogeneous catalyst for N-arylation of benzimidazole. Ind Eng Chem Res 50:9594–9600
Boström J, Hogner A, Llinàs A, Wellner E, Plowright AT (2012) Oxadiazoles in medicinal chemistry. J Med Chem 55:1817–1830
Dabiri M, Salehi P, Baghbanzadeh M, Bahramnejad M (2006) A facile procedure for the one-pot synthesis of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett 47:6983–6986
Dogan HN, Duran A, Rollas S, Sener G, Uysal MK, Gulen D (2002) Synthesis of new 2,5-disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg Med Chem 10:2893–2898
Flidallah HM, Sharshira EM, Basaif SA, A-Ba-Oum AEK (2002) Phosphorus Sulfur Silicon Relat Elem 177:67–79
Guin S, Ghosh T, Rout SK, Banerjee A, Patel BK (2011) Cu(II) catalyzed imine C-H functionalization leading to synthesis of 2,5-substituted 1,3,4-oxadiazoles. Org Lett 13:5976–5979
James ND, Growcott JW (2009) Zibotentan. Drugs Future 34:624–633
Jedlovska E, Lesko J (1994) A simple one-pot procedure for the synthesis of 1,3,4-oxadiazoles. Synth Commun 24:1879–1885
Kantam ML, Soumi L, Yadav J, Suresh B (2008) An efficient synthesis of propargylamines via three-component coupling of aldehydes, amines and alkynes catalyzed by nanocrystalline copper(II) oxide. Tetrahedron Lett 49:3083–3086
Kawano T, Yoshizumi T, Hirano K, Satoh T, Miura M (2009) Copper-mediated direct arylation of 1,3,4-oxadiazoles and 1,2,4-triazoles with aryl iodides. Org Lett 11:3072–3075
Kumar GS, Maheswari CU, Kumar RA, Kantam ML, Reddy KR (2011) Copper-catalyzed oxidative C-O coupling by direct C–H bond activation of formamides: synthesis of enol carbamates and 2-carbonyl-substituted phenol carbamates. Angew Chem Int Ed 50:11748–11751
Lee YZ, Chen X, Chen SA, Wei PK, Fann WS (2001) Soluble electroluminescent poly(phenylene vinylene)s with balanced electron- and hole injections. J Am Chem Soc 123:2296–2307
Liu K, Lu X, Zhang HJ, Sun J, Zhu HL (2012) Synthesis, molecular modeling, and biological evaluation of 2-(benzylthio)-5-aryloxadiazole derivatives as antitumor agents. Eur J Med Chem 47:473–478
Milcent R, Barbier GJ (1983) Oxydation d’hydrazones par le bioxyde de plomb: Nouvelles synthèses d’oxadiazoles-1,3,4 et de dérivés de l’amino-4 triazol-1,2,4 one-5. Heterocycl Chem 20:77–80
Okimoto M, Chiba T (1990) Electrochemical oxidation of ketone acylhydrazones and their hydrogen cyanide adducts in sodium cyanide-methanol. Transformation of ketones to nitriles. J Org Chem 55:1070–1076
de Oliveira CS, Lira BF, Barbosa-Filho JM, Lorenzo JGF, de Athayde-Filho PF (2012) Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: a review of the literature from 2000–2012. Molecules 17:10192–10231
Ouyang X, Piatnitski EL, Pattaropong V, Chen X, He HY, Kiselyov AS, Velankar A, Kawakami J, Labelle M, Smith L, Lohman J, Lee SP, Malikzay A, Fleming J, Gerlak J, Wang Y, Rosler RL, Zhou K, Mitelman S, Camara M, Surguladze D, Doody JF, Tuma MC (2006) Oxadiazole derivatives as a novel class of antimitotic agents: synthesis, inhibition of tubulin polymerization, and activity in tumor cell lines. Bioorg Med Chem Lett 16:1191–1196
Prakash O, Kumar M, Kumar R, Sharma C, Aneja KR (2010) Hypervalent iodine(III) mediated synthesis of novel unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles as antibacterial and antifungal agents. Eur J Med Chem 45:4252–4257
Prakash O, Hussain K, Kumar R, Deepak W, Sharma C, Aneja KR (2011) Synthesis and antimicrobial evaluation of new 1,4-dihydro-4-pyrazolylpyridines and 4-pyrazolylpyridines. Org Med Chem Lett 1:1–5
Pushpan P, Boja P, Chandrashekar C, Sunil Kumar B (2012) Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl)phenyl pyrazole moiety as cytotoxic agents. Eur J Med Chem 53:203–210
Reddy MA, Jain N, Yada D, Kishore C, Vangala JR, Surendra PR, Addlagatta A, Kalivendi SV, Sreedhar B (2011) Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J Med Chem 54:6751–6760
Rostamizadeh S, Housaini SAG (2004) Microwave assisted syntheses of 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett 45:8753–8756
Rout SK, Guin S, Nath J, Patel BK (2012) An “on-water” exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles. Green Chem 14:2491–2498
Saberi D, Heydari A (2013) CuO nanoparticles supported on α-Fe2O3-modified CNTs: a magnetically separable catalyst for oxidative C–O coupling of formamides with 1,3-dicarbonyl compounds. Tetrahedron Lett 54:4178–4180
Shi W, Qian X, Zhang R, Song G (2001) Synthesis and quantitative structure–activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles. J Agric Food Chem 49:124–130
Short FW, Long LM (1969) Synthesis of 5-aryl-2-oxazolepropionic acids and analogs antiinflammatory agents. J Heterocycl Chem 6:707–712
Singh S, Sharma LK, Saraswat A, Siddiqui IR, Kehrib HK, Singh RKP (2013) Electrosynthesis and screening of novel 1,3,4-oxadiazoles as potent and selective antifungal agents. RSC Adv 3:4237–4245
Stokes BJ, Driver TG (2011) Transition metal catalyzed formation of N-Heterocycles via aryl- or vinyl C–H bond amination. Eur J Org Chem 2011:4071–4088
Sugimoto Y, Konoki K, Murata M, Matsushita M, Kanazawa H, Oishi T (2009) Design, synthesis, and biological evaluation of fluorinated analogues of salicylihalamide. J Med Chem 52:798–806
Summa V, Petrocchi A, Bonelli F, Crescenzi B, Donghi M, Ferrara M, Fiore F, Gardelli C, Gonzalez Paz O, Hazuda DJ, Jones P, Kinzel O, Laufer R, Monteagudo E, Muraglia E, Nizi E, Orvieto F, Pace P, Pescatore G, Scarpelli R, Stillmock K, Witmer MV, Rowley M (2008) Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIVAIDS infection. J Med Chem 51:5843–5855
Werber G, Bucherri F, Noto R, Gentile M (1977) 1,5-dipolar cycloaddition reactions semicarbazone bromides, 5-alkyl (or aryl)amino-1,3,4-oxadiazole-2-carboxylic acids and their esters. J Heterocycl Chem 14:1385–1388
Zarghi A, Faizi M, Shafaghi B, Ahadian A, Khojastehpoor HR, Zanganeh V, Tabatabaia SA, Shafieec A (2005a) Design and synthesis of new 2-substituted-5-(2-benzylthiophenyl)-1,3,4-oxadiazoles as benzodiazepine receptor agonists. Bioorg Med Chem Lett 15:3126–3129
Zarghi A, Tabatabai SA, Faizi M, Ahadian A, Navabi P, Zanganeh V, Shafiee A (2005b) Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-1,3,4-oxadiazoles. Bioorg Med Chem Lett 15:1863–1865
Zhang M (2010) Copper catalyzed/mediated aromatic C–H bond functionalization. Appl Organomet Chem 24:269–284
Zhang ZM, Zhang XW, Zhao ZZ, Yan R, Xu R, Gong HB, Zhu HL (2012) Synthesis, biological evaluation and molecular docking studies of 1,3,4-oxadiazole derivatives as potential immunosuppressive agents. Bioorg Med Chem 20:3359–3367
Zhao J, Wang Y, He Y, Liu L, Zhu Q (2012) Cu-catalyzed oxidative C(sp2)–H cycloetherification of o-arylphenols for the preparation of dibenzofurans. Org Lett 14:1078–1081
Zovko A, Gabri MV, Kristina S, Pohleven F, Jakli D, Cimerman NG, Lu Z, Edrada-Ebel RA, Houssen WE, Mancini I, Defant A, Jaspars M, Turk T (2012) Antifungal and antibacterial activity of 3-alkylpyridinium polymeric analogs of marine toxins. Int Biodeterior Biodegrad 68:71–77
Acknowledgments
The authors are thankful to the Director, Indian Institute of Chemical Technology, Hyderabad for the encouragement, RP thankful to CSIR, New Delhi, India for the award of research fellowships. We thank CSIR for financial support under the 12th Five Year plan projects “Affordable Cancer Therapeutics (ACT)’’ (CSC 0301) and “Small Molecules in Lead Exploration (SMiLE)” (CSC0111).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murty, M.S.R., Penthala, R., Buddana, S.K. et al. Recyclable CuO nanoparticles-catalyzed synthesis of novel-2,5-disubstituted 1,3,4-oxadiazoles as antiproliferative, antibacterial, and antifungal agents. Med Chem Res 23, 4579–4594 (2014). https://doi.org/10.1007/s00044-014-1025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1025-x