Abstract
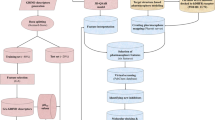
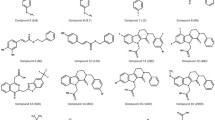
Retinoic acid receptor alpha (RARα) has been considered as one of the most important targets for the treatment of acute promyelocytic leukemia. To discover more novel lead compounds, ligand-based pharmacophore modeling of a series of structurally diverse RARα agonists was applied to acquire the binding model (KI pharmacophore model) and the efficacy model (EC50 pharmacophore model) of RARα. In this paper, a three-dimensional quantitative structure–activity relationship (3D-QSAR) in Discovery Studio 2.5 was used to generate pharmacophore models. Via Fischer’s randomization validation and maximum unbiased validation, the best pharmacophore model for KI pharmacophore model was Hypo1K and for EC50 pharmacophore model was Hypo7E. Virtual screening of National Cancer Institute database using Hypo1K and Hypo7E was performed, respectively. Six potent compounds in the retrieved hits with a CAS number were confirmed to be effective on leukemia cell lines and other tumors in the literatures. As evident from the validation and the biological screening results, it can be concluded that the Hypo1K and Hypo7E were reliable and useful tools for lead optimization of novel RARα agonists.









Similar content being viewed by others
References
Bachmair F, Hoffmann R, Daxenbichler G, Langew T (2000) Studies on structure–Activity relationships of retinoic acid receptor ligands by means of molecular modeling. Vitam Horm 59:159–215
Bardos TJ, Chmielewicz ZF, Hebborn P (1969) Structure-activity relationships of alkylating agents in cancer chemotherapy*. Ann N Y Acad Sci 163:1006–1025
Beard RL, Colon DF, Klein ES, Vorse KA, Chandraratna RAS (1995) Differential RXR & RAR activity of stilbene retinoid analogs bearing thiazole and imidazole carboxylic acids. Bioorg Med Chem Lett 5:2729–2734
Beard RL, Teng M, Colon DF, Duong TT, Thacher SM, Arefieg T, Chandraratna RAS (1997) Synthesis and biological activity of 1, 2, 3, 4-tetrahydroquinoline and 3, 4-(1H)-dihydroquinolin-2-one analogs of retinoic acid. Bioorg Med Chem Lett 7:2373–2378
Beard RL, Duong TT, Teng M, Klein ES, Standevan AM, Chandraratna RAS (2002) Synthesis and biological activity of retinoic acid receptor alpha specific amides. Bioorg Med Chem Lett 12:3145–3148
Benbrook DM, Subramanian S, Gale JB, Liu S, Brown CW, Boehm MF, Berlin KD (1998) Synthesis and characterization of heteroarotinoids demonstrate structure specificity relationships. J Med Chem 41:3753–3757
Boehm MF, McClurg MR, Pathirana C, Mangelsdorf D, White SK, Hebert J, Winn D, Goldman ME, Heyman RA (1994) Synthesis of high specific activity tritium-labeled [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties. J Med Chem 37:408–414
Charpentier B, Bernardon JM, Eustache J, Millois C, Martin B, Michel S, Shroot B (1995) Synthesis, structure-affinity relationships, and biological activities of ligands binding to retinoic acid receptor subtypes. J Med Chem 38:4993–5006
Cincinelli R, Dallavalle S, Nannei R, Carella S, De Zani D, Merlini L, Penco S, Garattini E, Giannini G, Pisano C, Vesci L, Carminati P, Zuco V, Zanchi C, Zunino F (2005) Synthesis and structure-activity relationships of a new series of retinoid-related biphenyl-4-ylacrylic acids endowed with antiproliferative and proapoptotic activity. J Med Chem 48:4931–4946
Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Brit J Pharmacol 125:924–947
Dawson MI, Jong L, Hobbs PD, Cameron JF, Chao WR, Pfahl M, Lee MO, Shroot B, Pfahl M (1995) Conformational effects on retinoid receptor selectivity. 2. Effects of retinoid bridging group on retinoid X receptor activity and selectivity. J Med Chem 38:3368–3383
de Lera AR, Bourguet W, Altucci L, Gronemeyer H (2007) Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6:811–820
Del Rio A, Barbosa AJ, Caporuscio F, Mangiatordi GF (2010) CoCoCo: a free suite of multiconformational chemical databases for high-throughput virtual screening purposes. Mol BioSyst 6:2122–2128
Dhapalapur MG, Sabnis SS, Deliwala CV (1968) Potential anticancer agents. II. Schiff bases from benzaldehyde nitrogen mustards. J Med Chem 11:1014–1019
Diaz P, Michel S, Stella L, Charpentier B (1997) Synthesis and biological activities of new heterocyclic aromatic retinoids. Bioorg Med Chem Lett 7:2289–2294
Evans DA, Doman TN, Thorner DA, Bodkin MJ (2007) 3D QSAR methods: phase and catalyst compared. J Chem Inf Model 47:1248–1257
Farmer LJ, Zhi L, Jeong S, Lamph WW, Osburn DL, Croston G, Flatten KS, Heyman RA, Nadzan AM (2003) Retinoic acid receptor ligands based on the 6-cyclopropyl-2, 4-hexadienoic acid. Bioorg Med Chem Lett 13:261–264
Grange EW, Henry DW, Lee WW (1980) Glyoxylic acid hydrocarbylsulfonylhydrazones and therapeutic compositions. US Patents 4,218,465
Haffner CD, Lenhard JM, Miller AB, McDougald DL, Dwornik K, Ittoop OR, Gampe RT Jr, Xu HE, Blanchard S, Montana VG, Consler TG, Bledsoe RK, Ayscue A, Groom D (2004) Structure-based design of potent retinoid X receptor alpha agonists. J Med Chem 47:2010–2029
Haraldsdóttir S, Guðlaugsdóttir E, Ingólfsdóttir K, Ögmundsdóttir HM (2004) Anti-proliferative effects of lichen-derived lipoxygenase inhibitors on twelve human cancer cell lines of different tissue origin in vitro. Planta Med 70:1098–1100
Howe LR (2007) Rexinoids and breast cancer prevention. Clin Cancer Res 13:5983–5987
Jiao W, Blunt JW, Cole ALJ, Munro MHG (2004) Fumagiringillin, a new Fumagillin derivative from a strain of the fungus Aspergillus fumigatus. J Nat Prod 67:1434–1437
Jong L, Lehmann JM, Hobbs PD, Harlev E, Huffman JC, Pfahl M, Dawson MI (1993) Conformational effects on retinoid receptor selectivity. 1. Effect of 9-double bond geometry on retinoid X receptor activity. J Med Chem 36:2605–2613
Koch SSC, Dardashti LJ, Hebert JJ, White SK, Croston GE, Flatten KS, Heyman RA, Nadzan AM (1996) Identification of the first retinoid X receptor homodimer antagonist. J Med Chem 39:3229–3234
Langer T, Hoffmann RD, Bachmair F, Begle S (2000) Chemical function based pharmacophore models as suitable filters for virtual 3D-database screening. J Mol Struct 503:59–72
Michellys PY, Ardecky RJ, Chen JH, D’Arrigo J, Grese TA, Karanewsky DS, Leibowitz MD, Liu S, Mais DA, Mapes CM, Montrose-Rafizadeh C, Ogilvie KM, Reifel-Miller A, Rungta D, Thompson AW, Tyhonas JS, Boehm MF (2003) Design, synthesis, and structure-activity relationship studies of novel 6, 7-locked-[7-(2-alkoxy-3,5-dialkylbenzene)-3-methylocta]-2,4,6-trienoic acids. J Med Chem 46:4087–4103
Miwako I, Kagechika H (2007) Tamibarotene. Drugs Today (Barc) 43:563–568
Muccio DD, Brouillette WJ, Breitman TR, Taimi M, Emanuel PD, Zhang XK, Chen GQ, Sani BP, Venepally P, Reddy L, Muzaffar Alam, Simpson-Herren L, Hill DL (1998) Conformationally defined retinoic acid analogues. 4. Potential new agents for acute promyelocytic and juvenile myelomonocytic leukemias. J Med Chem 41:1679–1687
Rohrer SG, Baumann K (2009) Maximum unbiased validation (MUV) data sets for virtual screening based on PubChem bioactivity data. J Chem Inf Model 49:169–184
Scanlon KJ, Moroson BA, Bertino JR, Hynes JB (1979) Quinazoline analogues of folic acid as inhibitors of thymidylate synthetase from bacterial and mammalian sources. Mol Pharmacol 16:261–269
Smellie A, Teig SL, Towbin P (1995) Poling: promoting conformational variation. J Comput Chem 16:171–187
Takayama N, Kizaki M, Hida T, Kinjo K, Ikeda Y (2001) Novel mutation in the PML/RARα chimeric gene exhibits dramatically decreased ligand-binding activity and confers acquired resistance to retinoic acid in acute promyelocytic leukemia. Exp Hematol 29:864–872
Takeuchi M (2006) Clinical experience with a new synthetic retinoid, tamibarotene (Am-80) for relapsed or refractory acute promyelocytic leukemia. Gan to kagaku ryoho Cancer Chemother 33:397–401
Umemiya H, Fukasawa H, Ebisawa M, Eyrolles L, Kawachi E, Eisenmann G, Gronemeyer H, Hashimoto Y, Shudo K, Kagechika H (1997) Regulation of retinoidal actions by diazepinylbenzoic acids. 1 retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem 40:4222–4234
Vuligonda V, Lin Y, Chandraratna RAS (1996) Synthesis of highly potent RXR-specific retinoids: the use of a cyclopropyl group as a double bond isostere. Bioorg Med Chem Lett 6:213–218
Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111:2505–2515
Wang GZ, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi PP (1998) Role of PML in cell growth and the retinoic acid pathway. Science 279:1547–1551
Zhang L, Nadzan AM, Heyman RA, Love DL, Mais DE, Croston G, Lamph WW, Boehm MF (1996) Discovery of novel retinoic acid receptor agonists having potent antiproliferative activity in cervical cancer cells. J Med Chem 39:2659–2663
Acknowledgments
The work was supported by the State Key Development Program for Basic Research of China (Grant No. 2009CB918501).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Li, Y., Cao, Z. et al. Binding affinity and efficacy-based pharmacophore modeling studies of retinoic acid receptor alpha agonists and virtual screening for potential agonists from NCI. Med Chem Res 23, 3916–3926 (2014). https://doi.org/10.1007/s00044-014-0939-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-0939-7