Abstract
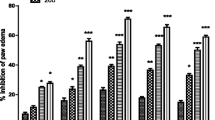
Pedilanthus tithymaloides (PT) leaves are widely used in Indian medicine to treat inflammation and pain, but until recently no systematic study of these activities were reported. This study aimed to evaluate the antiinflammatory, antinociceptive, and antipyretic activity of the chloroform (CE) and methanol extract (ME) of PT leaves and its isolated constituents in animal models. The results revealed significant antiinflammatory activity of CE and ME in carrageenan-induced paw edema (acute), vascular permeability and cotton pellet granuloma (chronic) models. Both extracts also produced significant increase in hot-plate reaction time but decrease in writhing response in a dose-dependent manner, indicating their antinociceptive activity. Moreover, significant antipyretic activity was recorded in yeast-induced pyrexia model. Chemical study of CE and ME yielded five known compounds, namely epifriedelanyl acetate (1), friedelanol (2), β-sitosterol (3), ursolic acid (4), and luteolin (5), along with the new compound 1,2-tetradecanediol 1-(hydrogen sulfate) sodium salt (6). This is the first report showing isolation of compound 5 from PT leaves, and of compound 6, from any plant source. Among these, compounds 4 and 5 (10 mg/kg, p.o.) produced significant inhibition of pain response elicited by acetic acid, increased latency in hot-plate, and caused inhibition of carrageenan-induced edema and cotton pellet-induced granuloma formation in mice. Thus, our results clearly demonstrated the justification of the traditional use of P. tithymaloides leaves as an antiinflammatory drug in primary health care.






Similar content being viewed by others
References
Abreu P, Matthew S, Gonzalez T, Costa D, Segundo MA, Fernandes E (2006) Anti-inflammatory and antioxidant activity of a medicinal tincture from Pedilanthus tithymaloides. Life Sci 78:1578–1585
Abreu MP, Matthew S, Gonzalez T, Vanickova L, David C, Gomes A, Marcela AS, Fernandes E (2008) Isolation and identification of antioxidants from Pedilanthus tithymaloides. J Nat Med 62:67–70
Akhlaq M, Mohammed A (2011) New phenolic acids from the galls of Tamarixaphylla (L.) Karst. Int Res J Pharm 4:222–225
Al-Ghamdi MS (2001) The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol 76:45–48
Ankush RD, Nagda KK, Suryakar AN, Ankush NR (2003) Tuberculosis altering erythrocyte membrane topology—a lectin haemagglutination study. Indian J Tuberc 50:95–98
Antonisamy P, Ignacimuthu S (2010) Immunomodulatory, analgesic and antipyretic effects of violacein isolated from Chromobacterium violaceum. Phytomedicine 17:300–304
Arigoni ME (1977) Prostaglandins: possible mechanism of anti-inflammatory drugs. Inflammation and anti-inflammatories. Spectrum Publication, New York, pp 119–120
Basu A, Chaudhuri AK (1991) Preliminary studies on the anti-inflammatory and analgesic activities of Calotropis procera root extract. J Ethnopharmacol 31:319–324
Begum S, Saxena B, Goyal M, Ranjan R, Vijaya R, Joshi B, Rao ChV, Krishnamurthy S, Sahai M (2009) Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamusniger and isolation of a new coumarinolignan. Fitoterapia 81:178–184
Burch RM, DeHaas CH (1990) A bradykinin antagonist inhibits carrageenan edema in rats. Naunyn Schmiedebergs Archiv Pharmacol 342:189–193
Bush IE, Alexander RW (1960) An improved method for the assay of anti-inflammatory substances in rats. Acta Endocr 35:268–276
Chiruvella KK, Mohammed A, Dampuri G, Ghanta GR, Raghavan CS (2007) Phytochemical and antimicrobial studies of methylangolensate and luteolin-7-o-glucoside isolated from callus cultures of Soymidafebrifuga. Int J Biomed Sci 3:269–278
Choi JH, Jung BH, Kang OH, Choi HJ, Park PS, Cho SH, Kim YC, Kim YC, Sohn DH, Park H, Lee JH, Kwon DY (2006) The anti-inflammatory and antinociceptive effects of ethyl acetate fraction of Cynanchipaniculata radix. Biol Pharm Bull 29:971–975
Dai Y, Hang BQ, Tan LW (1989) An anti-inflammatory effect of oleanolic acid. Chin J Pharmacol Toxicol 3:96–99
D’Arcy PD, Howard EM, Muggleton PW, Townsend SB (1960) The antiinflammatory action of griseofulvin in experimental animals. J Pharm Pharmacol 12:659–665
Deraedt R, Jougney S, Delevalcee F, Falhout M (1980) Release of prostaglandin E and F in analgogenic reaction and its inhibition. Eur J Pharmacol 51:17–24
Dhar SN, Ray SM, Roy A, Dutta SK (1988) Oral anti-inflammatory activity of pedilanthain—a new proteolytic enzyme from Pedilanthus tithymaloides Poit. Indian J Pharm Sci 50:281–283
Eddy NB, Leimbach D (1953) Synthetic analgesics: II. Dithienylbutenyl and dithienyl-butylamines. J Pharmacol Exp Ther 107:385–393
Ghosh S, Samanta A, Mandal NB, Bannerjee S, Chattopadhyay D (2012) Evaluation of the wound healing activity of methanol extracts of Pedilanthus tithymaloides (L.) Poit leaf and its isolated active constituents in topical formulation. J Ethnopharmacol 142:714–722
Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X (2006) Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J Nutr 136:1517–1521
Hicks R (1969) The evaluation of inflammation induced by material implanted subcutaneously in the rat. J Pharm Pharmacol 21:581–588
Hirobe C, Qiao ZS, Takeya K, Itokawa H (1997) Cytotoxic flavonoids from Vitexagnus-castus. Phytochemistry 46:521–524
Hunskaar S, Hole K (1987) The formalin tests in mice: dissociation between inflammatory and noninflammatory pain. Pain 30:103–104
Jang S, Kelley KW, Johnson RW (2008) Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 105:7534–7539
Keith MW, Sally AL, Michael WS, Thomas JG, Garry MM (1990) Needles contain amounts of taxol comparable to the stem bark of Taxus brevifolia: analysis and isolation. J Nat Prod 53:1249–1255
Koster R, Anderson M, De-Beer EJ (1959) Acetic acid for analgesic screening. Fed Proc 18:412–418
Kumar A, Kulkarni SK (2006) Protective effect of BR-16A, a polyherbal preparation against social isolation stress: possible GABAergic mechanism. J Phytother Res 20:538–541
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53:597–652
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–135
Mahato SB, Kundu A (1994) 13C NMR spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry 37:1517–1575
Mahato SB, Sarkar SK, Poddar G (1988) Triterpenoid saponins. Phytochemistry 27:3037–3067
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Misra DR, Khastgir HN (1969) Terpenoids and related compounds: chemical investigation of Pedilanthus tithymaloides. J Indian Chem Soc 66:843–844
Mukherjee KS, Laha S, Manna TK, Chakraborty CK (1992) Chemical investigation on Limnophilarogusa and Pedilanthus tithymaloides. J Indian Chem Soc 69:411–412
Nagda KK, Deshmukh B (1998) Haemagglutination pattern of galactose specific lectin from Pedilanthus tithymaloides in diabetes mellitus. Indian J Exp Biol 36:426–428
Najid A, Simon A, Cook J, Chable-Rabinovitch H, Delage C, Chulia AJ, Rigaud M (1992) Characterization of ursolic acid as a lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and differentiated HL 60 leukemic cells. Fed Eur Biochem Soc 299:213–217
Northover BJ (1963) The permeability to plasma proteins of the peritoneal blood vessels of the mouse, and the effect of substances that alter permeability. J Pathol Bacteriol 85:361–370
Odontuya G, Hoult JRS, Houghton PJ (2005) Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother Res 19:782–786
Oku H, Ishiguro K (2002) Cyclooxygenase-2 inhibitory 1,4-naphthoquinones from Impatiens balsamina L. Biol Pharm Bull 25:658–660
Osawa T, Kawakishi S, Namiki M (1990) Anti-mutagenesis and anti-carcinogenesis mechanism II. Plenum Press, New York, pp 139–153
Panthong A, Supraditaporn W, Kanjanapothi D, Taesotikul T, Reutrakul V (2007) Analgesic, anti-inflammatory and venotonic effects of Cissusquadrangularis Linn. J Ethnopharmacol 110:264–270
Parada CA, Tambeli CH, Cunha FQ, Ferreira SH (2001) The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience 102:937–944
Pettit GR, Ducki S, Tan R, Gardella SR, McMahon BJ, Boyd RM, Pettit RG, Blumberg MP, Lewin EN, Doubek LD, Williams DM (2002) Isolation and structure of pedilstain from a Republic of Maldives Pedilanthus Sp. J Nat Prod 65:1262–1265
Phan VK, Chau VM, Hoang TH, Nguyen HN, Jung JL, Young HK (2004) Pentacyclic triterpenoids from Mallotus apelt. Arch Pharmacal Res 27:1109–1113
Piepmeyer JA (1966) Dry-cleaning detergent compositions. US 3254029 19660531
Renne EP (1996) The pregnancy that doesn’t stay: the practice and perception of abortion by Ekiti Yoruba women. Soc Sci Med 42:483–494
Seshagirirao K (1995) Purification and partial characterization of a lectin from Pedilanthus tithymaloides latex. Biochem Arch 11:197–201
Simon A, Najid A, Chulia AJ, Delage C, Rigaud M (1992) Inhibition of lipoxygenase activity and HL60 leukemic cell proliferation by ursolic acid isolated from heather flowers (Calluna vulgaris). Biochem Biophys Acta 1125:68–72
Sulaiman MR, Perimal EK, Akhtar MN, Mohamad AS, Khalid MH, Tasrip NA, Mokhtar F, Zakaria ZA, Lajis NH, Israf DA (2010) Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 81:855–858
Swingle KF, Shideman FE (1972) Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain antiinflammatory agents. J Pharmacol Exp Ther 183:226
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51:5–17
Vane JR, Botting RM (1998) Anti-inflammatory drugs and their mechanism of action. Inflamm Res 47:S78–S87
Vidotti JG, Zimmermann A, Sarragiotto MH, Nakamura CV, Benedito P, Filho D (2006) Antimicrobial and phytochemical studies on Pedilanthus tithymaloides. Fitoterapia 77:43–46
Whittle BA (1964) The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol 2:246–253
Winter CA, Risley EA, Nuss GW (1962) Carrageenan induced oedema in hind paw of rat as assay for antiinflammatory drugs. Exp Biol Med 111:544–547
Zhou C, Sun X, Liu W, Shi H, Gao H, Miao Y (1993) Effects of oleanolic acid on the immune complex allergic reaction and inflammation. J Clin Pharmacol Sci 2:69–79
Acknowledgments
The authors are grateful to the Indian Council of Medical Research, New Delhi for providing Senior Research Fellowship to one of the authors (SG), and the Department of Pharmaceutical Technology, Jadavpur University, Kolkata for providing all the necessary facilities. Authors are also thankful to the Scientists of Division of Natural Product Chemistry, Indian Institute of Chemical Biology, Jadavpur, Kolkata, for their help in isolation and spectral data interpretation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, S., Chattopadhyay, D., Mandal, A. et al. Bioactivity guided isolation of antiinflammatory, analgesic, and antipyretic constituents from the leaves of Pedilanthus tithymaloides (L.). Med Chem Res 22, 4347–4359 (2013). https://doi.org/10.1007/s00044-012-0449-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0449-4