Abstract
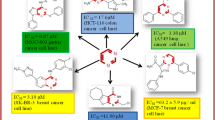
Condensation of iminodiacetic acid 1 with various amines i.e., cyclohexanamine, 1-(3-aminopropyl)imidazole, pyridin-2-ylmethanamine, pyridin-3-ylmethanamine, pyridin-4-ylmethanamine, 2-morpholinoethanamine, thiophen-2-ylmethanamine, 2-(thiophen-2-yl)ethanamine, furan-2-ylmethanamine, 2-(pyrrolidin-1-yl)ethanamine, and 1-(3-aminopropyl) pyrrolidin-2-one 2a–k under microwave irradiation gave the corresponding piperazine-2,6-dione derivatives 3a–k in quantitative yields. Piperazine-2,6-dione derivatives 3a–k on condensation with 1H-indole-2-carboxylic acid under microwave irradiation gave the corresponding 4-(1H-indole-2-carbonyl)piperazine-2,6-dione derivatives 4a–k in quantitative yields. All the synthesized compounds (3a–k & 4a–k) were purified by crystallization and characterized by spectroscopic means. On screening at a concentration of 10 μM, compounds 3k, 4e, 4i breast (T47D), 4j lung (NCI H-522), 3i colon (HCT-15), 4e ovary (PA-1), and 4g liver (HepG-2) exhibited good anticancer activity.

Similar content being viewed by others
References
Abraham WR (2005) Controlling pathogenic gram-negative bacteria by interfering with their biofilm formation. Drug Des Rev 2:13–33
Boger DL, Fink BE, Hedrick MP (2000) A new class of highly cytotoxic diketopiperazines. Bioorg Med Chem Lett 10:1019–1020
Bryans J, Charlton P, Chicarelli-Robinson I, Collins M, Faint R, Latham C, Shaw I, Trew S (1996) Inhibition of plasminogen activator inhibitor-1 activity by two diketopiperazines, XR330 and XR334 produced by streptomyces sp. J Antibiot 49:1014–1021
Cody WL, Augelli-Szafran CE, Berryman KA, Cai C, Doherty AM, Edmunds JJ, He JX, Narasimhan LS, Penvose-Yi J, Plummer JS, Rapundalo ST, Rubin JR, Van Huis CA, Leblond L, Winocour PD, Siddiqui MA (1999) The design of potent and selective inhibitors of thrombin utilizing a piperazinedione template: part 2. Bioorg Med Chem Lett 9:2503–2508
Folkes A, Roe MB, Sohal S, Golec J, Faint R, Brooks T, Charlton P (2001) Synthesis and in vitro evaluation of a series of diketopiperazine inhibitors of plasminogen activator inhibitor-1. Bioorg Med Chem Lett 11:2589–2592
Grauslund M, Thougaard AV, Fuchtbauer A, Hofland KF, Hjorth PH, Jensen PB, Sehested M, Fuchtbauer EM, Jensen LH (2007) A mouse model for studying the interaction of bisdioxopiperazines with topoisomerase IIα in vivo. Mol Pharmacol 72:1003–1014
Hazra A, Paira P, Palit P, Banerjee S, Mondal NB, Sahu NP (2007) Synthesis of symmetrically 1,4-disubstituted piperazine-2,5-diones: a new class of antileishmanial agents. J Chem Res 2007:381–383
Houston DR, Synstad B, Eijsink VGH, Stark MJR, Eggleston IM, Van ADMF (2004) Structure-based exploration of cyclic dipeptide chitinase Inhibitors. J Med Chem 47:5713–5720
Jhaumeer-Laulloo S, Khodabocus A, Jugoo A, Jheengut D, Sobha S (2003) Synthesis of diketopiperazines containing prolinyl unit-cyclo(l-prolinyl-l-leucine), cyclo(l-prolinyl-l-isoleucine) and cyclo(l-tryptophyl-l-proline). J Indian Chem Soc 80:765–768
Loughlin WA, Marshall RL, Carreiro A, Elson KE (2000) Solution-phase combinatorial synthesis and evaluation of piperazine-2,5-dione derivatives. Bioorg Med Chem Lett 10:91–94
Martins MB, Carvalho I (2007) Diketopiperazines: biological activity and synthesis. Tetrahedron 63:9923–9932
Molesworth PP, Gardiner MG, Jones RC, Smith JA, Tegg RS, Wilson C (2010) Synthesis and phytotoxicity of structural analogues of thaxtomin natural products. Aust J Chem 63:813–820
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P (1991) Feasibility of a high–flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766
Ong CW, Chang YA, Jing-Yun Wu, Chien-Chung Cheng (2003) Novel design of a pentacyclic scaffold as structural mimic of saframycin A. Tetrahedron 59:8245–8249
Pons JF, Fauchere JL, Lamaty F, Molla A, Lazaro R (1998) A constrained diketopiperazine as a new scaffold for the synthesis of peptidomimetics. Eur J Org Chem 5:853–859
Poster DS, Penta JS, Bruno S, MacDonald JS (1981) ICRF-187 in clinical oncology. Cancer Clin Trials 4:143–146
Shvedaite IP, Udrenaite EB, Lauzhikene NA, Gaidyalis PG (1999) Synthesis and anti-inflammatory activity of 4-acyl and 4-sulfonyl derivatives of piperazine-2,6-dione. Pharm Chem J 33:313–316
Sinha S, Srivastava R, De Clercq E, Singh RK (2004) Synthesis and antiviral properties of arabino and ribonucleosides of 1,3-dideazaadenine, 4-nitro-1, 3-dideazaadenine and diketopiperazine. Nucleosides Nucleotides Nucleic Acids 23:1815–1824
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Sondhi SM, Rani R, Gupta PP, Agrawal SK, Saxena AK (2009a) Synthesis, anticancer, and anti-inflammatory activity evaluation of methanesulfonamide and amidine derivatives of 3,4-diaryl-2-imino-4-thiazolines. Mol Divers 13:357–366
Sondhi SM, Rani R, Roy P, Agrawal SK, Saxena AK (2009b) Microwave-assisted synthesis of N-substituted cyclic imides and their evaluation for anticancer and anti-inflammatory activities. Bioorg Med Chem Lett 19:1534–1538
Sondhi SM, Rani R, Singh J, Roy P, Agarwal SK, Saxena AK (2010a) Solvent free synthesis, anti-inflammatory and anticancer activity evaluation of tricyclic and tetracyclic benzimidazole derivatives. Bioorg Med Chem Lett 20:2306–2310
Sondhi SM, Singh J, Rani R, Gupta PP, Agarwal SK, Saxena AK (2010b) Synthesis, anti- inflammatory and anticancer activity evaluation of some novel acridine derivatives. Eur J Med Chem 45:555–563
Sondhi SM, Rani R, Roy P, Agrawal SK, Saxena AK (2011) Synthesis, anti-inflammatory, and anticancer activity evaluation of some heterocyclic amidine and bis-amidine derivatives. J Heterocycl Chem 48:921–926
Sondhi SM, Singh J, Agrawal SK, Saxena AK, Roy P (2012) Synthesis of pyrimidine and condensed pyrimidine derivatives and their evaluation for anti-inflammatory activity. Med Chem Res 21:91–99
Toshiharu N, Shinichi Y, Toshihiko K, Muneaki T, Akira H, Makoto I, Shigeru T (1990) Antitumor activity of MST-16, a novel derivative of bis(2,6-dioxopiperazine), in murine tumor models. Cancer Chemother Pharmacol 26:193–197
Tuntiwachwuttikul P, Taechowisan T, Wanbanjob A, Thadaniti S, Taylor WC (2008) Lansai A-D, secondary metabolites from Streptomyces sp. SUC1. Tetrahedron 64:7583–7586
Acknowledgments
We are thankful to the technical staff of the Chemistry Department, I. I. T. Roorkee, for spectroscopic studies and elemental analysis and also to Head I. I. C. for providing NMR facility. Special thanks are due to Prof. G. Bhattacharjee and Prof. Ravi Bhushan of Chemistry Department, I. I. T. Roorkee, for helpful discussion. Mr. Sandeep Kumar is thankful to MHRD, New Delhi for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Kumar, N., Roy, P. et al. Efficient synthesis of piperazine-2,6-dione and 4-(1H-indole-2-carbonyl)piperazine-2,6-dione derivatives and their evaluation for anticancer activity. Med Chem Res 22, 4600–4609 (2013). https://doi.org/10.1007/s00044-012-0438-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0438-7