Abstract
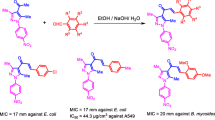
We synthesized 36 chalcone-like (E)-3-(substitutedphenyl)-1-hetrylprop-2-en-1-ones by condensing 2-acetylfuran/2-acetylpyrrole with substituted benzaldehydes under basic conditions. Of the 36 molecules synthesized, 10 are new to the literature. Bio-evaluation studies of these molecules revealed that compounds 5, 9, 15, 25, and 29 were potent NorA efflux pump inhibitors against Staphylococcus aureus by reducing MIC of ciprofloxacin fourfold, while compounds 11, 21, 25, and 26 showed promising anticancer activity in all four tested cancer cell lines (HL-60, MOLT-4, PC-3, and HeLa). Compound 25 emerged as a very good potentiator of ciprofloxacin against multidrug resistant S. aureus and also showed promising anticancer activity. The present communication describes syntheses, bio-evaluation, and structure-related activity of the (E)-3-(substitutedphenyl)-1-hetrylprop-2-en-1-ones.





Similar content being viewed by others
References
Achanta G, Modzelewska A, Feng L, Khan SR, Huang P (2006) A Boronic-chalcone derivative exhibits potent anticancer activity through inhibition of the proteasome. Mol Pharmacol 70:426–433
Bandgar BP, Gawande SS, Bodade RG, Totre JV, Khobragade CN (2010) Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg Med Chem 18:1364–1370
Belofsky G, Percivill D, Lewis K, Tegos GP, Ekart J (2004) Phenolic metabolic of Dalea versicolor that enhance antibiotic activity against multi-drug resistant bacteria. J Nat Prod 67:481–484
Bremner PD, Meyer JJ (1998) Pinocembrin chalcone: an antibacterial compound from Helichrysum trilineatum. Planta Med 64:777
Bsasaif SA, Sobahi TR, Khalil AK, Hassan MA (2005) Stereoselective crossed-aldol condensation of hetarylmethyl ketones with aromatic aldehydes in water: synthesis of (2E)-3-Aryl-1-hetarylprop-2-en-1-ones. Bull Korean Chem Soc 26:1677–1681
Echeverria C, Santibanez JF, Donoso-Tauda O, Escobar CA, Tagle RR (2009) Structural antitumoral activity relationships of synthetic chalcones. Int J Mol Sci 10:221–231
Farrugia LJ (1997) ORTEP-3 for Windows—a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Cryst 30:565
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst 32:837–838
Forejtníková H, Lunerová K, Kubínová R, Jankovská D, Marek R, Kares R, Suchý V, Vondrácek J, Machala M (2005) Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology 208:81–93
Friss-Möller A, Chen M, Fuursted K, Christensen SB, Kharazmi A (2002) In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med 68:416–419
Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T (2002) Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci 71:1449–1463
Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T (2000) Phenolic constituents of licorice. viii. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull 48:1286–1292
Hijova E (2006) Bioavailability of chalcones. Bratisl Lek Listy 107:80–84
Ilango K, Valentina P, Saluja G (2010) Synthesis and In vitro anti-cancer activity of some substituted Chalcone derivatives. Res J Pharm Biol Chem Sci 1:354–359
Jasinski JP, Butcher RJ, Narayana B, Veena K, Yathirajan HS (2009) (2E)-1-(3-Chlorophenyl)-3-(4-chlorophenyl)prop-2-en-1-one. Acta Cryst E65:2641–2642
Joshi AS, Li XC, Nimrod AC, ElSohly HN, Walker LA, Clark AM (2001) Dihydrochalcones from Piper longicaudatum. Planta Med 67:186–188
Kaatz GW, Seo SM (1995) Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother 39:2650–2655
Kaatz GW, Seo SM, Ruble CA (1993) Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother 37:1086–1094
Kaatz GW, Seo SM, Foster T (1999) Introduction of a norA promoter region mutation into the chromosome of a fluoroquinolone-susceptible strain of Staphylococcus aureus using plasmid integration. Antimicrob Agents Chemother 43:2222–2224
Kromann H, Larsen M, Boesen T, SchØnning K, Nielsen SF (2004) Synthesis of prenylated benzaldehydes and their use in the synthesis of analogues of licochalcone A. Eur J Med Chem 3:1000–9993
Lawrence NJ, Patterson RP, Ooi LL, Cook D, Ducki S (2006) Effects of alpha-substitutions on structure and biological activity of anticancer chalcones. Bioorg Med Chem Lett 16:5844–5848
Li DQ (2008) 3-(4-Methoxyphenyl)-1-(2-pyrrolyl)prop-2-en-1-one. Acta Cryst E64:1920
Li XZ, Nikaido H (2004) Efflux-mediated drug resistance in bacteria. Drugs 64:159–204
Memurry LM, Petrucci REJ, Levy SB (1980) Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci 77:3974–3977
Mustafa K, Kjaergaard HG, Perry NB, Weavers RT (2003) Hydrogen-bonded rotamers of 2′,4′,6′-trihydroxy-3′-formyldihydrochalcone, an intermediate in the synthesis of a dihydrochalcone from Leptospermum recurvum. Tetrahedron 59:6113–6120
Nardelli M (1995) PARST95 - an update to PARST: a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J Appl Cryst 28:659
Neyfakh AA, Borsch CM, Kaatz GW (1993) Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother 37:128–129
Nielsen SF, Larsen M, Boesen T, SchØnning K, Kromann H (2005) Cationic chalcone antibiotics. Design, synthesis, and mechanism of action. J Med Chem 48:2667–2677
Poole KJ (2005) Efflux-mediated antimicrobial resistance. J Antimicrob Chemother 56:20–51
Prasad YR, Kumar PR, Ramesh B (2007) Synthesis and antidepressant activity of some new 3-(2′′-hydroxy naphthalen-1′′-yl)-5-phenyl-2-Isoxazolines. Int J Chem Sci 5:542–548
Radwan MAA, Abbas EMH (2009) Synthesis of some pyridine, thiopyrimidine, and isoxazoline derivatives based on the pyrrole moiety. Monatsh Chem 140:229–233
Rateb NM, Zohdi HF (2009) Atom-efficient, solvent-free, green synthesis of chalcones by grinding. Synth Commun 39:2789–2794
Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A (2004) Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428:198
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Preti D (2008) Design, synthesis, and biological evaluation of thiophene analogues of chalcones. Bioorg Med Chem 16:5367–5376
Sheldrick GM (1997) SHELX97 University of Gottingen, Germany
Szliszka E, Czuba ZP, Mazur B, Sedek L, Paradysz A, Krol W (2010) Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int J Mol Sci 11:1–13
Tang SP, Kuang DZ, Feng YL, Li W, Chen ZM (2008) 3-[4-(Dimethyl-amino)phenyl]-1-(2-pyrrolyl)prop-2-en-1-one. Acta Cryst E64:1123
Tsukiyama RI, Katsura H, Tokuriki N, Kobayashi M (2002) Antibacterial activity of Licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother 46:1226–1230
Wong E (1968) The role of chalcones and flavanones in flavonoid biosynthesis. Phytochemistry 7:1751–1758
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Kumar, S., Ali, F. et al. Synthesis and biologic activities of some novel heterocyclic chalcone derivatives. Med Chem Res 22, 3969–3983 (2013). https://doi.org/10.1007/s00044-012-0401-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0401-7