Abstract
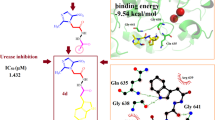
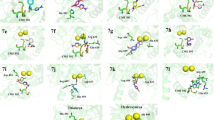
A series of novel hybrid molecules containing sulfanilamide and thiourea templates was designed and synthesized. These compounds were screened for antimicrobial and urease inhibitory activities. Some of the compounds revealed promising antibacterial and antifungal activities. Fascinatingly, the majority of the compounds exhibited potential urease inhibitory activities with IC50 ranging from 0.20 to 7.50 μM. Compound 2b was identified as the most potent urease inhibitor (IC50 0.20 μM), and was 100-fold more potent than thiourea, the standard inhibitor. Molecular docking studies of compounds were performed on 3D crystal structures of Jack bean and Helicobacter pylori ureases.











Similar content being viewed by others
References
Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT (2004) Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett 14(1):217–223
Al-Rashida M, Ashraf M, Hussain B, Nagra SA, Abbas G (2011) Discovery of new chromone containing sulfonamides as potent inhibitors of bovine cytosolic carbonic anhydrase. Bioorg Med Chem 19(11):3367–3371
Aly AA, Brown AB, Ramadan M, Abdel-Aziz M, Abuo-Rahma GE-DAA, Radwan MF, Gamal-Eldeen AM (2010) Selectivity of N-aroyl-N′-arylthioureas towards 2-(1,3-dioxo-1H-inden-2(3H)-ylidene)malononitrile. New synthesis of (Z)-N-((E)-4-amino-1-aryl-5-cyano-6-oxo-1H-indeno[1,2-d][1,3]- thiazepin-2(6H)-ylidene)-4-arylamides of antitumor and antioxidant activities. J Heterocycl Chem 47(3):503–508. doi:10.1002/jhet.344
Aslam MA, Mahmood SU, Shahid M, Saeed A, Iqbal J (2011) Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur J Med Chem 46(11):5473–5479
Bestmann HJ, Kern F, Schäfer D, Witschel MC (1992) 3,4-Dihydroisocoumarins, a new class of ant trail pheromones. Angew Chem Int Ed Engl 31(6):795–796. doi:10.1002/anie.199207951
Dixon NE, Gazzola TC, Blakeley RL, Zermer B (1975) Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 97(14):4131–4133
Drews J (2000) Drug discovery: a historical perspective. Science 287(5460):1960–1964 8361 [pii]
Faidallah HM, Albar HA, Makki MSI, Sharshira EM (2002) Synthesis of novel cyclic benzenesulfonylurea and thiourea derivatives. Phosphorus Sulfur Silicon Relat Elem 177(3):685–693. doi:10.1080/10426500210275
Gould IM (2008) The epidemiology of antibiotic resistance. Int J Antimicrob Agents 32(Suppl 1):S2–S9
Gribble FM, Reimann F (2003) Differential selectivity of insulin secretagogues: mechanisms, clinical implications, and drug interactions. J Diabetes Complications 17(2 Suppl):11–15
Hanif M, Saleem M, Hussain MT, Rama NH, Zaib S, Aslam MAM, Jones PG, Iqbal J (2012a) Synthesis, urease inhibition, antioxidant and antibacterial studies of some 4-amino-5-aryl-3H-1,2,4-triazole-3-thiones and their 3,6-disubstituted 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole derivatives. J Braz Chem Soc 23:854–860
Hanif M, Shoaib K, Saleem M, Hasan Rama N, Zaib S, Iqbal J (2012b) Synthesis, urease inhibition, antioxidant, antibacterial, and molecular docking studies of 1,3,4-oxadiazole derivatives. ISRN Pharmacol 2012:928901. doi:10.5402/2012/928901
Klingenstein R, Melnyk P, Leliveld SR, Ryckebusch A, Korth C (2006) Similar structure-activity relationships of quinoline derivatives for antiprion and antimalarial effects. J Med Chem 49(17):5300–5308. doi:10.1021/jm0602763
Koch KR (2001) New chemistry with old ligands: N-alkyl- and N,N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord Chem Rev 216–217:473–488. doi:10.1016/s0010-8545(01)00337-x
Kontoyiannis DP, Mantadakis E, Samonis G (2003) Systemic mycoses in the immunocompromised host: an update in antifungal therapy. J Hosp Infect 53(4):243–258
Krungkrai J, Scozzafava A, Reungprapavut S, Krungkrai SR, Rattanajak R, Kamchonwongpaisan S, Supuran CT (2005) Carbonic anhydrase inhibitors. Inhibition of Plasmodium falciparum carbonic anhydrase with aromatic sulfonamides: towards antimalarials with a novel mechanism of action? Bioorg Med Chem 13(2):483–489
Labute P (2007) Protonate 3D: assignment of macromolecular protonation state and geometry. Chemical Computing Group Inc., Montreal
Maren TH (1976) Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 16:309–327. doi:10.1146/annurev.pa.16.040176.001521
Mobley HL, Hausinger RP (1989) Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev 53(1):85–108
Mobley HL, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59(3):451–480
MOE (2010) The molecular operating environment version 201010. Chemical Computing Group Inc., Montreal. http://wwwchemcompcom
Montecucco C, Rappuoli R (2001) Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol 2(6):457–466. doi:10.1038/35073084
Nair PC, Sobhia ME (2008) Quantitative structure activity relationship studies on thiourea analogues as influenza virus neuraminidase inhibitors. Eur J Med Chem 43(2):293–299
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66(7):1022–1037. doi:10.1021/np030096l
Nitta T, Arai T, Takamatsu H, Inatomi Y, Murata H, Iinuma M, Tanaka T, Ito T, Asai F, Ibrahim I, Nakanishi T, Watabe K (2002) Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J Health Sci 48(3):273–276. doi:10.1248/jhs.48.273
Ramadas K, Suresh G, Janarthanan N, Masilamani S (1998) Antifungal activity of 1,3-disubstituted symmetrical and unsymmetrical thioureas. Pestic Sci 52(2):145–151. doi:10.1002/(sici)1096-9063(199802)52:2<145:aid-ps677>3.0.co;2-j
Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF (2006) Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med 6:2
Ryckebusch A, Deprez-Poulain R, Debreu-Fontaine MA, Vandaele R, Mouray E, Grellier P, Sergheraert C (2002) Parallel synthesis and anti-malarial activity of a sulfonamide library. Bioorg Med Chem Lett 12(18):2595–2598
Saczewski F, Kuchnio A, Samsel M, Lobocka M, Kiedrowska A, Lisewska K, Saczewski J, Gdaniec M, Bednarski PJ (2010) Synthesis of novel aryl(heteroaryl)sulfonyl ureas of possible biological interest. Molecules 15(3):1113–1126
Saeed A, Erben MF, Flörke U (2010) Effect of fluorine substitution on the crystal structures and vibrational properties of phenylthiourea isomers. J Mol Struct 982(1–3):91–99. doi:10.1016/j.molstruc.2010.08.012
Saeed A, Mumtaz A, Ishida H (2011) Synthesis, characterization of some new 1-aroyl-3-(4-aminosulfonylphenyl)thioureas and crystal structure of 1-(3,4,5-trimethoxybenzoyl)- 3-(4-aminosulfonylphenyl)thiourea. J Sulfur Chem 32(1):45–54. doi:10.1080/17415993.2010.541461
Sieradzki K, Roberts RB, Haber SW, Tomasz A (1999) The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 340(7):517–523. doi:10.1056/NEJM199902183400704
Thornber CW (1979) Isosterism and molecular modification in drug design. Chem Soc Rev 8(4):563–580
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39(8):971–974. doi:10.1021/ac60252a045
Yonova PA, Stoilkova GM (2004) Synthesis and biological activity of urea and thiourea derivatives from 2-aminoheterocyclic compounds. J Plant Growth Regul 23(4):280–291. doi:10.1007/s00344-003-0054-3
Zampini IC, Cuello S, Alberto MR, Ordonez RM, D’Almeida R, Solorzano E, Isla MI (2009) Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J Ethnopharmacol 124(3):499–505
Acknowledgments
This study was financially supported by COMSTECH–TWAS and German-Pakistani Research Collaboration Programme to JI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeed, A., Zaib, S., Pervez, A. et al. Synthesis, molecular docking studies, and in vitro screening of sulfanilamide-thiourea hybrids as antimicrobial and urease inhibitors. Med Chem Res 22, 3653–3662 (2013). https://doi.org/10.1007/s00044-012-0376-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0376-4