Abstract
Epidemiological studies have established an inverse relationship between plasma high-density lipoprotein (HDL) cholesterol concentration, and incidence of coronary artery disease (CAD); thus, the development of novel therapies that attempt to exploit the atheroprotective functions of HDL is a major goal. Inhibition of cholesteryl ester transfer protein (CETP) is one of the approaches targeted to increase HDL cholesterol concentration. CETP is a glycoprotein involved in transporting lipoprotein particles and neutral lipids between HDL and low-density lipoproteins (LDL), and therefore CETP inhibitors could be useful agents in the future for treating dyslipidemia and related disorders. Guided by our previously reported pharmacophore and QSAR models for CETP inhibition, we synthesized and bioassayed a series of sulfonic acid ester and benzenesulfonamide derivatives that can serve as a promising lead compounds for the development of potential and selective CETP inhibitors. The most potent compound 6k illustrated an IC50 of 3.4 μM.
Similar content being viewed by others
Introduction
Cardiovascular disease continues to be a leading cause of death worldwide. Dyslipidemia, a major risk factor for cardiovascular disease, represents a multifactorial disease and current antidyslipidemic medicines focus on either lowering low-density lipoproteins (LDL) cholesterol or raising high-density lipoprotein (HDL) cholesterol (Grass et al., 1995; Paigen et al., 1990; Hansson, 2005).
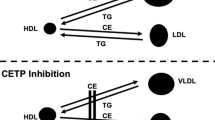
A number of epidemiological studies have established an inverse relationship between serum HDL cholesterol levels and the incidence of ischemic heart disease (Julve et al., 2011; Lamarche et al., 1996). HDL removes excess cholesterol from peripheral tissues to the liver for biliary elimination (Cuchel et al., 2010; Lewis, 2006; Tall et al., 2001). CETP, a 476-residue glycoprotein, is engaged in interchanging lipoprotein particles and neutral lipids, including cholesteryl esters, phospholipids, and triglycerides between HDL and LDL (Chapman et al., 2010). CETP, as revealed by X-ray crystallography (PDB code: 2OBD, resolution 2.2 Å), has a lipophilic binding site capable of binding up to four lipid molecules (Qiu et al., 2007). In human plasma, CETP plays a proatherogenic task by moving cholesteryl esters from HDL to very low-density lipoprotein (VLDL) and LDL particles, thereby lowering atheroprotective HDL cholesterol and raising proatherogenic VLDL and LDL cholesterols. Obviously, the risk of coronary artery disease (CAD) is proportional to the plasma levels of CETP (Vasan et al., 2009; Boekholdt et al., 2004). Actually, It is rather frequent within the CAD population to have elevated CETP plasma protein levels that are 2- to 3-fold higher than concentrations typically found in the plasma of normal subjects (1–3 μg/ml) (McPherson et al., 1991).
Indication exists that the outcomes of CETP activity may depend on the metabolic setting, particularly on triglyceride levels. Therefore, pharmacological CETP inhibition may reduce the risk of CAD in humans, but only in those with high triglyceride levels (Vasan et al., 2009; Boekholdt et al., 2004).
The inaccessibility of a reasonable high-resolution crystallographic structures for CETP combined with its large binding pocket locked up most modeling-related discovery projects to ligand-based approaches particularly quantitative structure–activity relationship analysis (QSAR) (Castilho et al., 2007; Hanumantharao et al., 2005; Kelkar et al., 2004; Cronin and Schultz, 2003; Akamatsu, 2002).
Earlier, we have developed ligand-based three-dimensional (3D) pharmacophores integrated within self-consistent QSAR model for CETP inhibitors. The pharmacophore models were used as 3D search queries to mine 3D libraries for new CETP inhibitors, while the QSAR model predicted their biological activities and therefore prioritize them for in vitro evaluation (Abu Khalaf et al., 2010; Abu Sheikha et al., 2010). Our shape-constrained Hypo4/8 captured the most potent hit (1) with IC50 value of 1.9 μM (see Fig. 1) (Abu Khalaf et al., 2010).
However, optimization based upon the pharmacophoric features of hit 1 and the lipophilic binding site of CETP target guided us to design and synhesize a novel series of derivatives of N-(4-benzyloxyphenyl)-4-methyl-benzenesulfonamide (6a–6g, Scheme 1), N-(4-benzyloxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide (6h–6l, Scheme 1), N-(4-benzylaminophenyl)-toluene-4-sulfonic acid ester (8a–8c, Scheme 2), and 4-(N,N)-[bis-(benzylaminophenyl)]-toluene-4-sulfonic acid ester (8d–8l, Scheme 2) that have CETP inhibitory activities. In the newly synthesized compounds, we explore the electronic effect of different substituents such as CF3, Br, NO2, Cl, OCH3, and CH3 together with the compound lipophilicity effect on the CETP inhibitory activity.
Materials and methods
General methods
The proposed structures for compounds 6a–6l and 8a–8l were confirmed via elemental analyses, IR spectroscopy, mass spectroscopy, 1H- and 13C-NMR spectra.
Melting points were measured using Gallenkampf melting point apparatus and are uncorrected. 1H- and 13C-NMR spectra were collected on a Varian Oxford NMR300 spectrometer. The samples were dissolved in CDCl3. Mass spectrometry was performed using LC Mass Bruker Apex-IV mass spectrometer utilizing an electrospray interface.
Infrared spectra were recorded using Shimadzu IRAffinity-1 spectrophotometer. The samples were dissolved in CHCl3 and analyzed as thin solid films using NaCl plates. Analytical thin layer chromatography (TLC) was carried out using pre-coated aluminum plates and visualized by UV light (at 254 and/or 360 nm). Elemental analysis was performed using EuroVector elemental analyzer.
Chemicals and solvents were purchased from corresponding companies (Sigma-Aldrich, Riedel-de Haen, Fluka, BDH Laboratory Supplies and Promega Corporation) and were used in the experimentation without further purification.
General procedure for the synthesis of N-(4-benzyloxyphenyl)-4-methyl-benzenesulfonamide and N-(4-benzyloxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide derivatives (6a–6l)
Tosyl chloride 2 (0.953 g, 5 mmol) was dissolved in CH2Cl2 (10 ml) in an ice bath and p-aminophenol 3 (0.655 g, 6 mmol) was added. Then, triethylamine (0.83 ml, 6 mmol) was added. The mixture was stirred at room temperature for 2 h and then extracted with water (10 ml). The organic extract was dried on anhydrous Na2SO4 and filtered. The residue, after evaporation of the solvent, was purified by column chromatography eluting with CH2Cl2/MeOH (95:5) to give N-(4-hydroxy-phenyl)-4-methyl-benzenesulfonamide 4a (0.397 g, 30%) and 4b (0.167 g, 16%).
Subsequently, N-(4-hydroxy-phenyl)-4-methyl-benzenesulfonamide 4a (0.741 g, 2.8 mmol) was dissolved in DMF (5 ml) in an ice bath and one of the substituted benzyl bromides 5a–5i (3.6 mmol) was added. Then, NaOH (0.24 g, 6 mmol) was added. The mixture was stirred at room temperature for 3 h and then DMF was evaporated. The residue was dissolved in CH2Cl2 (10 ml) and extracted with water (10 ml). The organic extract was dried on anhydrous Na2SO4 and filtered. The residue, after evaporation of the solvent, was purified by column chromatography eluting with CH2Cl2/cyclohexane (85:15) to give N-(4-benzyloxyphenyl)-4-methyl-benzenesulfonamide derivatives 6a–6g.
In addition, N-(4-hydroxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide 4b (1.169 g, 2.8 mmol) was dissolved in DMF (5 ml) in an ice bath and one of the substituted benzyl bromides 5a–5i (3.6 mmol) was added. Then, NaOH (0.24 g, 6 mmol) was added. The mixture was stirred at room temperature for 3 h and then DMF was evaporated. The residue was dissolved in CH2Cl2 (10 ml) and extracted with water (10 ml). The organic extract was dried on anhydrous Na2SO4 and filtered. The residue, after evaporation of the solvent, was purified by column chromatography eluting with CH2Cl2/cyclohexane (85:15) to give N-(4-benzyloxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide derivatives 6h–6l.
N-(4-Hydroxy-phenyl)-4-methyl-benzenesulfonamide (4a)
Offwhite powder (30%): R f = 0.77 (CHCl3–MeOH, 95:5); mp. 162–163°C; 1H-NMR (300 MHz, CDCl3) δ 10.3 (s, 1H, OH), 7.62 (d, J = 8.25 Hz, 2H), 7.40 (d, J = 8.33 Hz, 2H), 6.95 (dd, J = 8.94, 3.0 Hz, 2H), 6.85 (dd, J = 8.96, 2.98 Hz, 2H), 2.35 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.25 (1C), 145.53 (1C), 137.32 (1C), 131.68 (1C), 130.21 (2C), 127.16 (2C), 123.41 (2C), 121.51 (2C), 21.66 ppm (1C); IR (thin film) cm−1 3465, 3021, 2963, 1721, 1603, 1501, 1377, 1215.
N-(4-Hydroxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide (4b)
White powder (16%): R f = 0.85 (CHCl3–MeOH, 95:5); mp. 150–151°C; 1H-NMR (300 MHz, CDCl3) δ 10.4 (s, 1H, OH), 7.59 (d, J = 8.34 Hz, 2H), 7.44 (d, J = 8.28 Hz, 2H), 6.95–7.10 (m, 4H), 6.75–6.89 (m, 4H), 2.43 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.68 (1C), 145.65 (1C), 134.50 (1C), 134.31 (1C), 131.91 (2C), 131.30 (2C), 129.80 (2C), 129.72 (2C), 128.54 (2C), 128.19 (2C), 123.03 (2C), 21.78 (1C), 21.65 ppm (1C); IR (thin film) cm−1 3512, 3027, 2959, 1725, 1601, 1500, 1368, 1221.
4-Methyl-N-[4-(4-trifluoromethyl-benzyloxy)-phenyl]-benzenesulfonamide (6a)
White powder (33%): R f = 0.85 (CHCl3–MeOH, 98:2); mp. 115–116°C; 1H-NMR (300 MHz, CDCl3) δ 7.58 (d, J = 7.82 Hz, 2H), 7.48 (dd, J = 12.09, 6.96 Hz, 2H), 7.25 (m, 4H), 7.85 (m, 2H), 6.72 (d, J = 8.75 Hz, 1H), 6.42 (d, J = 8.76 Hz, 1H), 4.70 (s, 2H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 148.76 (1C), 145.71 (1C), 144.18 (1C), 140.03 (1C), 137.53 (1C), 135.13 (1C), 129.94 (2C), 129.76 (2C), 127.47 (2C), 125.43(2C), 123.08 (2C), 113.06 (2C), 54.21 (1C), 26.96 (1C), 21.74 ppm (1C); IR (thin film) cm−1 3399, 3032, 2963, 1597, 1497, 1454; MS (ESI, positive mode) m/z [M + Na]+ 444.08517 (C21H18F3NNaO3S requires 444.43377); Anal. Calcd for C21H18F3NO3S: C 59.85, H 4.31, F 13.52, N 3.32, S 7.61, found: C 59.57, H 4.93, F 13.52, N 3.25, S 7.81.
N-[4-(4-Bromo-benzyloxy)-phenyl]-4-methyl-benzenesulfonamide (6b)
Off-white powder (63%): R f = 0.75 (CHCl3); mp. 129–130°C; 1H-NMR (300 MHz, CDCl3) δ 7.66 (d, J = 8.27 Hz, 2H), 7.42 (d, J = 8.32 Hz, 2H), 7.28 (d, J = 8.25 Hz, 2H), 7.18 (d, J = 8.28 Hz, 2H), 6.73 (dd, J = 6.89, 1.94 Hz, 2H), 6.43 (dd, J = 6.95, 1.86 Hz, 2H), 4.23 (s, 2H), 2.45 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.46 (1C), 145.13 (1C), 141.20 (1C), 137.80 (1C), 132.52 (1C), 131.82 (2C), 129.68 (2C), 129.11 (2C), 128.62 (2C), 123.32 (2C), 121.20 (1C), 113.19 (2C), 47.86 (1C), 21.79 ppm (1C); IR (thin film) cm−1 3399, 3030, 2967, 1605, 1520, 1489, 1474; MS (ESI, positive mode) m/z [M + Na]+ 455.00830 (C20H18BrNNaO3S requires 455.33186); Anal. Calcd for C20H18BrNO3S: C 55.56, H 4.20, Br 18.48, N 3.24, S 7.42, found: C 55.86, H 4.37, Br 18.48, N 3.06, S 7.31.
4-Methyl-N-[4-(4-methyl-benzyloxy)-phenyl]-benzenesulfonamide (6c)
Off-white powder (15%): R f = 0.82 (CHCl3–MeOH, 98:2); mp. 119–120°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.27 Hz, 2H), 7.28 (d, J = 8.30 Hz, 2H), 7.15 (dd, J = 13.19, 8.01 Hz, 4H), 6.72 (dd, J = 8.92, 3.34 Hz, 2H), 6.45 (dd, J = 8.93, 1.99 Hz, 2H), 4.20 (s, 2H), 2.45 (s, 3H), 2.30 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.92 (1C), 145.01 (1C), 140.99 (1C), 137.17 (1C), 135.86 (1C), 132.64 (1C), 129.64 (2C), 129.42 (2C), 128.64 (2C), 127.56 (2C), 123.24 (2C), 113.02 (2C), 48.28 (1C), 21.75 (1C), 21.14 ppm (1C); IR (thin film) cm−1 3422, 3041, 2924, 1605, 1508, 1447; MS (ESI, positive mode) m/z [M + Na]+ 390.11344 (C21H21NNaO3S requires 390.46238); Anal. Calcd for C21H21NO3S: C 68.64, H 5.76, N 3.81, S 8.73, found: C 68.64, H 5.70, N 3.67, S 8.64.
4-Methyl-N-[4-(4-nitro-benzyloxy)-phenyl]-benzenesulfonamide (6d)
Yellow powder (12%): R f = 0.63 (CHCl3); mp. 118–119°C; 1H-NMR (300 MHz, CDCl3) δ 8.25 (d, J = 7.95 Hz, 2H), 7.78 (d, J = 7.47 Hz, 2H), 7.55 (d, J = 8.02 Hz, 2H), 7.30 (d, J = 7.5 Hz, 2H), 6.78 (d, J = 8.27 Hz, 2H), 6.45 (d, J = 7.97 Hz, 2H), 4.48 (s, 2H), 2.43 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.32 (1C), 146.66 (1C), 146.03 (1C), 145.20 (1C), 141.46 (1C), 132.53 (1C), 129.70 (2C), 129.31 (2C), 127.81 (2C), 124.00 (2C), 123.43 (2C), 113.27 (2C), 47.83 (1C), 21.78 ppm (1C); IR (thin film) cm−1 3395, 3063, 2843, 1605, 1516, 1474; MS (ESI, positive mode) m/z [M + Na]+ 421.08286 (C20H18N2NaO5S requires 421.43340); Anal. Calcd for C20H18N2O5S: C 60.29, H 4.55, N 7.03, S 8.05, found: C 60.40, H 4.25, N 6.94, S 8.54.
N-[4-(3-Chloro-benzyloxy)-phenyl]-4-methyl-benzenesulfonamide (6e)
White oil (13.6%): R f = 0.82 (CHCl3–MeOH, 98:2); 1H-NMR (300 MHz, CDCl3) δ 7.7 (d, J = 8.31 Hz, 2H), 7.15–7.40 (m, 6H), 6.7 (dd, J = 6.83, 2.19 Hz, 2H), 6.4 (dd, J = 6.86, 2.2 Hz, 2H), 4.15 (s, 2H), 2.4 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.60 (1C), 145.09 (1C), 141.15 (1C), 141.13 (1C), 134.62 (1C), 132.56 (2C), 130.01 (2C), 129.67 (2C), 128.62 (2C), 127.56 (1C), 127.39 (1C), 125.42 (1C), 123.30 (2C), 113.05 (2C), 47.85 (1C), 21.75 ppm (1C); IR (thin film) cm−1 3426, 3063, 2924, 1601, 1512, 1431, 1177; MS (ESI, positive mode) m/z [M + Na]+ 410.05881 (C20H18ClNNaO3S requires 410.06959).
N-[4-(4-Chloro-benzyloxy)-phenyl]-4-methyl-benzenesulfonamide (6f)
White powder (30.4%): R f = 0.82 (CHCl3–MeOH, 98:2); mp. 120–121°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.82 Hz, 2H), 7.20-7.32 (m, 6H), 6.72 (dd, J = 8.93, 2.08 Hz, 2H), 6.43 (dd, J = 8.95, 2.08 Hz, 2H), 4.25 (s, 2H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.61 (1C), 145.11 (1C), 141.12 (1C), 137.35 (1C), 133.12 (1C), 132.53 (1C), 129.68 (2C), 128.86 (1C), 128.80 (1C), 128.78 (2C), 128.62 (2C), 123.30 (2C), 113.08 (2C), 47.76 (1C), 21.79 ppm (1C); IR (thin film) cm−1 3399, 3032, 2963, 1605, 1512, 1493, 1447, 1173; MS (ESI, positive mode) m/z [M + H]+ 388.07687 (C20H19ClNO3S requires 388.06959); Anal. Calcd for C20H18ClNO3S: C 61.93, H 4.68, Cl 9.14, N 3.61, S 8.27, found: C 60.14, H 4.84, Cl 9.14, N 3.32, S 8.91.
N-[4-(3-Methoxy-benzyloxy)-phenyl]-4-methyl-benzenesulfonamide (6g)
Off-white powder (17.6%): R f = 076 (CHCl3–MeOH, 98:2); mp. 97–98°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.32 Hz, 2H), 7.18–7.31 (m, 4H), 6.78–6.92 (m, 2H), 6.72 (dd, J = 8.93, 2.13 Hz, 2H), 6.44 (dd, J = 8.96, 2.19 Hz, 2H), 4.26 (s, 2H), 3.80 (s, 3H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 159.98 (1C), 146.90 (1C), 145.03 (1C), 141.02 (1C), 140.53 (1C), 132.62 (1C), 129.78 (1C), 129.65 (2C), 128.64 (2 C), 123.25 (2C), 119.71 (1C), 113.12 (1C), 113.02 (2C), 112.73 (1C), 55.27 (1C), 48.44 (1C), 21.75 ppm (1C); IR (thin film) cm−1 3422, 3027, 2963, 1601, 1512, 1454, 1173; MS (ESI, positive mode) m/z [M + H]+ 384.12641 (C21H22NO4S requires 384.11913); Anal. Calcd for C21H21NO4S: C 65.78, H 5.52, N 3.65, S 8.36, found: C 65.36, H 5.62, N 3.27, S 8.15.
4-Methyl-N-[4-(3-trifluoromethyl-benzyloxy)-phenyl]-N-(toluene-4-sulfonyl)-benzenesulfonamide (6h)
Off-white oil (29.5%): R f = 0.85 (CHCl3–MeOH, 98:2); 1H-NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.32 Hz, 2H), 7.46 (d, J = 8.27 Hz, 3H), 7.35 (m, 3H), 7.25 (m, 4H), 6.85 (m, 4H), 4.75 (s, 2H), 2.43 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.88 (1C), 145.55 (1C), 144.07 (1C), 137.59 (1C), 136.90 (1C), 135.18 (1C), 132.28 (1C), 131.81 (1C), 129.97 (2C), 129.69 (2C), 129.02 (1C), 128.44 (2C), 127.72 (2C), 125.17 (2C), 125.12 (2C), 124.65 (1C), 124.60 (1C), 122.98 (2C), 54.28 (1C), 21.60 (1C), 21.52 ppm (1C); IR (thin film) cm−1 3032, 2963, 1597, 1497, 1451, 1161; MS (ESI, positive mode) m/z [M + Na]+ 598.09609 (C28H24F3NNaO5S2 requires 598.10480).
4-Methyl-N-[4-(3-chloro-benzyloxy)-phenyl]-N-(toluene-4-sulfonyl)-benzenesulfonamide (6i)
White oil (48.8%): R f = 0.79 (CHCl3–MeOH, 98:2); 1H-NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.35 Hz, 2H), 7.46 (d, J = 8.29 Hz, 2H), 7.22–7.30 (m, 4H), 7.05–7.18 (m, 4H), 6.78–6.90 (m, 4H), 4.60 (s, 2H), 2.40 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.73 (1C), 145.64 (1C), 144.08 (1C), 137.69 (1C), 134.90 (1C), 134.31 (1C), 131.91 (2C), 130.0 (2C), 129.80 (2C), 129.78 (2C), 129.72 (2C), 128.54 (2C), 128.50 (2C), 128.01 (1C), 126.69 (1C), 123.03 (2C), 54.12 (1C), 21.78 (1C), 21.65 ppm (1C); IR (thin film) cm−1 3032, 2924, 1597, 1497, 1449, 1157; MS (ESI, positive mode) m/z [M + Na]+ 564.06795 (C27H24ClNNaO5S2 requires 564.07844).
4-Methyl-N-[4-(4-chloro-benzyloxy)-phenyl]-N-(toluene-4-sulfonyl)-benzenesulfonamide (6j)
White powder (51.5%): R f = 0.85 (CHCl3-MeOH, 98:2); mp. 119.5-120.5°C; 1H-NMR (300 MHz, CDCl3) δ 7.56 (d, J = 8.32 Hz, 2H), 7.45 (d, J = 8.26 Hz, 2H), 7.22–7.30 (m, 4H), 7.05–7.18 (m, 4H), 6.78–6.84 (m, 4H), 4.60 (s, 2H), 2.44 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.70 (1C), 145.67 (1C), 144.04 (1C), 137.47 (1C), 134.90 (1C), 134.31 (1C), 130.07 (2C), 130.0 (2C), 129.76 (2C), 129.72 (2C), 129.65 (2C), 128.72 (2C), 128.66 (2C), 128.01 (1C), 127.71 (1C), 122.99 (2C), 54.03 (1C), 21.79 (1C), 21.67 ppm (1C); IR (thin film) cm−1 3028, 2963, 1597, 1497, 1447, 1262; MS (ESI, positive mode) m/z [M + Na]+ 564.05881 (C27H24ClNNaO5S2 requires 564.07844); Anal. Calcd for C27H24ClNO5S2: C 59.82, H 4.46, Cl 6.54, N 2.58, S 11.83, found: C 59.07, H 5.05, Cl 6.14, N 2.19, S 11.37.
4-Methyl-N-[4-(3-methyl-benzyloxy)-phenyl]-N-(toluene-4-sulfonyl)-benzenesulfonamide (6k)
White powder (32.1%): R f = 0.82 (CHCl3–MeOH, 98:2); mp. 125.5–126.5°C; 1H-NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.36 Hz, 2H), 7.33–7.43 (m, 6H), 6.82–7.12 (m, 8H), 4.65 (s, 2H), 2.35 (s, 6H), 2.15 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 148.12 (1C), 146.37 (1C), 144.27 (1C),137.98 (1C), 137.95 (1C), 136.24 (1C), 134.86 (1C), 131.42 (2C), 130.62 (2C), 130.31(1C), 130.29 (1C), 129.13 (2C), 128.70 (1C), 128.64 (3C), 127.83 (2C), 125.71 (1C), 122.89 (2C), 53.54 (1C), 21.68 (1C), 21.56 (1C), 21.41 ppm (1C); IR (thin film) cm−1 3028, 2955, 1597, 1497, 1451, 1165; MS (ESI, positive mode) m/z [M + Na]+ 544.12091 (C28H27NNaO5S2 requires 544.13306); Anal. Calcd for C28H27NO5S2: C 64.47, H 5.22, N 2.69, S 12.29, found: C 64.70, H 5.28, N 3.49, S 11.64.
4-Methyl-N-[4-(3-methoxy-benzyloxy)-phenyl]-N-(toluene-4-sulfonyl)-benzenesulfonamide (6l)
White powder (18.0%): R f = 0.88 (CHCl3–MeOH, 98:2); mp. 148–149°C; 1H-NMR (300 MHz, CDCl3) δ 7.55 (d, J = 8.28 Hz, 2H), 7.47 (d, J = 8.22 Hz, 2H), 7.08–7.28 (m, 6H), 6.68–6.89 (m, 6H), 4.60 (s, 2H), 3.74 (s, 3H), 2.43 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 159.66 (1C), 148.57 (1C), 145.57 (1C), 143.90 (1C), 137.00 (1C), 135.04 (1C), 131.89 (1C), 130.04 (2C), 129.75 (2C), 129.67 (2C), 129.44 (2C), 128.53 (2C), 127.73 (2C), 122.88 (2C), 120.91 (1C), 113.88 (1C), 113.45 (1C), 55.22 (1C), 54.56 (1C), 21.79 (1C), 21.66 ppm (1C); IR (thin film) cm−1 3029, 2924, 1597, 1497, 1458, 1165; MS (ESI, positive mode) m/z [M + Na]+ 560.10835 (C28H27NNaO6S2 requires 560.12798); Anal. Calcd for C28H27NO6S2: C 62.55, H 5.06, N 2.61, S 11.93, found: C 61.85, H 5.03, N 2.39, S 12.04.
General procedure for synthesis of N-(4-benzylaminophenyl)-toluene-4-sulfonic acid ester and 4-(N,N)-[bis-(benzylaminophenyl)]-toluene-4-sulfonic acid ester derivatives (8a–8l)
Tosyl chloride 2 (0.953 g, 5 mmol) was dissolved in CH2Cl2 (10 ml) in an ice bath and p-aminophenol 3 (0.655 g, 6 mmol) was added. Then, triethylamine (0.83 ml, 6 mmol) was added. The mixture was stirred at room temperature for 2 h and then extracted with water (10 ml). The organic extract was dried on anhydrous Na2SO4 and filtered. The residue, after evaporation of the solvent, was purified by column chromatography eluting with CH2Cl2/MeOH (95:5) to give toluene-4-sulfonic acid 4-amino-phenyl ester 7 (0.503 g, 38%).
Subsequently, 4-aminophenyl-4-toluene-sulfonic acid ester 7 (0.741 g, 2.8 mmol) was dissolved in DMF (5 ml) in an ice bath and one of the substituted benzyl bromides 5b–5h, 5j, and 5k (3.6 mmol) was added. Then, NaOH (0.24 g, 6 mmol) was added. The mixture was stirred at room temperature for 3 h and then DMF was evaporated. The residue was dissolved in CH2Cl2 (10 ml) and extracted with water (10 ml). The organic extract was dried on anhydrous Na2SO4 and filtered. The residue, after evaporation of the solvent, was purified by column chromatography eluting with CH2Cl2/cyclohexane (85:15) to give N-(4-benzylaminophenyl)-toluene-4-sulfonic acid ester and 4-(N,N)-[bis-(benzylaminophenyl)]-toluene-4-sulfonic acid ester derivatives 8a–8l.
4-Aminophenyl-4-toluene-sulfonic acid ester (7)
White powder (38%): R f = 0.80 (CHCl3–MeOH, 95:5); mp. 140–141°C; 1H-NMR (300 MHz, CDCl3) δ 7.64 (d, J = 8.33 Hz, 2H), 7.41 (d, J = 8.01 Hz, 2H), 6.57 (dd, J = 8.90, 2.19 Hz, 2H), 6.38 (dd, J = 8.94, 2.19 Hz, 2H), 5.18 (br s, 2H, NH 2 ), 2.38 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 148.37 (1C), 145.80 (1C), 139.47 (1C), 132.22 (1C), 130.50 (2C), 128.73 (2C), 122.96 (2C), 114.39 (2C), 21.64 ppm (1C); IR (thin film) cm−1 3378, 3021, 2967, 1721, 1610, 1505, 1424, 1215.
Toluene-4-sulfonic acid 4-(3-methyl-benzylamino)-phenyl ester (8a)
Off-white oil (14.9%): R f = 0.85 (CHCl3–MeOH, 98:2); 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.31 Hz, 2H), 7.05–7.32 (m, 6H), 6.75 (dd, J = 8.91, 2.12 Hz, 2H), 6.44 (dd, J = 8.94, 2.14 Hz, 2H), 4.27 (s, 2H), 3.96 (br s, 1H, NH), 2.44 (s, 3H), 2.30 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.04 (1C), 145.03 (1C), 140.93 (1C), 138.77 (1C), 138.46 (1C), 132.61 (1C), 129.65 (2C), 128.65 (3C), 128.31 (1C), 128.22 (1C), 124.59 (1C), 123.25 (2C), 112.93 (2C), 48.47 (1C), 21.76 (1C), 21.47 ppm (1C); IR (thin film) cm−1 3426, 3028, 2924, 1605, 1512, 1470, 1451; MS (ESI, positive mode) m/z [M + H]+ 368.13149 (C21H22NO3S requires 368.12421).
Toluene-4-sulfonic acid 4-(3-bromo-benzylamino)-phenyl ester (8b)
Off-white oil (26%): R f = 0.82 (CHCl3–MeOH, 98:2); 1H-NMR (300 MHz, CDCl3) δ 7.66 (d, J = 8.31 Hz, 2H), 7.48 (s, 1H), 7.38 (d, J = 7.49 Hz, 1H), 7.28 (d, J = 8.05 Hz, 2H), 7.24 (d, J = 6.24 Hz, 1H), 7.19 (m, 1H), 6.74 (dd, J = 8.98, 2.18 Hz, 2H), 6.43 (dd, J = 8.98, 2.15 Hz, 2H), 4.10 (br s, 1H, NH), 4.30 (s, 2H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 146.57 (1C), 145.08 (1C), 141.37 (1C), 141.17 (1C), 132.54 (1C), 130.52 (1C), 130.32 (2C), 129.67 (2C), 128.64 (2C), 125.90 (1C), 123.33 (2C), 122.86 (1C), 113.04 (2C), 47.81 (1C), 21.77 ppm (1C); IR (thin film) cm−1 3422, 3059, 2924, 1605, 1508, 1483; MS (ESI, positive mode) m/z [M + Na]+ 455.00830 (C20H18BrNNaO3S requires 455.33186).
Toluene-4-sulfonic acid 4-(3-nitro-benzylamino)-phenyl ester (8c)
Pale green oil (18.2%): R f = 0.76 (CHCl3); 1H-NMR (300 MHz, CDCl3) δ 7.67 (d, J = 8.26 Hz, 2H), 7.25–7.53 (m, 6H), 6.82 (d, J = 9.07 Hz, 2H), 6.64 (d, J = 9.03 Hz, 2H), 4.95 (br s, 1H, NH), 4.50 (s, 2H), 2.44 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 148.24 (1C), 145.42 (1C), 140.64 (1C), 139.23 (1C), 138.78 (1C), 132.18 (1C), 129.88 (2C), 129.74 (2C), 128.60 (1C), 127.49 (1C), 122.66 (2C), 121.95 (2C), 110.93 (2C), 47.46 (1C), 21.77 ppm (1C); IR (thin film) cm−1 3449, 3071, 2924, 1601, 1528, 1508, 1346; MS (ESI, positive mode) m/z [M + H]+ 399.16944 (C20H19N2O5S requires 399.09364).
Toluene-4-sulfonic acid 4-[bis-(4-bromo-benzyl)-amino]-phenyl ester (8d)
White powder (50%): R f = 0.82 (CHCl3); mp. 129–130°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.27 Hz, 2H), 7.42 (d, J = 8.36 Hz, 4H), 7.25 (d, J = 8.04 Hz, 2H), 7.05 (d, J = 8.31 Hz, 4H), 6.73 (d, J = 9.14 Hz, 2H), 4.52 (s, 4H), 2.45 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.43 (1C), 145.16 (1C), 140.93 (2C), 136.75 (2C), 132.68 (1C), 131.90 (4C), 129.69 (2C), 128.57 (4C), 128.43 (1C), 123.26 (2C), 121.01 (2C), 113.19 (2C), 54.22 (2C), 21.79 ppm (1C); IR (thin film) cm−1 3051, 2963, 1601, 1508, 1455; MS (ESI, positive mode) m/z [M + H]+ 602.97937 (C27H24Br2NO3S requires 602.35046); Anal. Calcd for C27H23Br2NO3S: C 53.93, H 3.86, Br 26.57, N 2.33, S 5.33, found: C 53.15, H 3.48, Br 26.48, N 2.62, S 5.64.
Toluene-4-sulfonic acid 4-[bis-(4-methyl-benzyl)-amino]-phenyl ester (8e)
Off-white powder (19%): R f = 0.82 (CHCl3–MeOH, 98:2); mp. 149–150°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.06 Hz, 2H), 7.26 (d, J = 9.74 Hz, 2H), 7.08 (dd, J = 8.79, 7.94 Hz, 8H), 6.70 (d, J = 8.98 Hz, 2H), 6.53 (d, J = 9.04 Hz, 2H), 4.55 (s, 4H), 2.45 (s, 3H), 2.30 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.13 (1C), 144.98 (1C), 140.82 (1C), 136.70 (2C), 134.95 (2C), 132.97 (1C), 129.64 (2C), 129.64 (4C), 128.60 (2C), 126.56 (4C), 123.01 (2C), 112.69 (2C), 54.19 (2C), 21.76 (1C), 21.13 ppm (2C); IR (thin film) cm−1 3062, 2974, 1601, 1501, 1462; MS (ESI, positive mode) m/z [M + Na]+ 494.17604 (C29H29NNaO3S requires 494.61150); Anal. Calcd for C29H29NO3S: C 73.86, H 6.20, N 2.97, S 6.80, found: C 73.94, H 6.36, N 2.63, S 6.23.
Toluene-4-sulfonic acid 4-[bis-(4-nitro-benzyl)-amino]-phenyl ester (8f)
Pale-green oil (61%): R f = 0.61 (CHCl3); 1H-NMR (300 MHz, CDCl3) δ 8.18 (d, J = 8.7 Hz, 4H), 7.68 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.62 Hz, 4H), 7.28 (d, J = 8.05 Hz, 2H), 6.76 (dd, J = 9.15, 2.17 Hz, 2H), 6.53 (dd, J = 9.18, 2.13 Hz, 2H), 4.68 (s, 4H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.42 (1C), 146.73 (1C), 145.30 (2C), 141.50 (2C), 132.62 (2C), 129.74 (2C), 128.51 (2C), 127.38 (4C), 124.21 (4C), 123.57 (2C), 113.32 (2C), 54.68 (2C), 21.79 ppm (1C); IR (thin film) cm−1 3055, 2963, 1597, 1512, 1478; MS (ESI, positive mode) m/z [M + Na]+ 556.11513 (C27H23N3NaO7S requires 556.55354).
Toluene-4-sulfonic acid 4-[bis-(3-trifluoromethyl-benzyl)-amino]-phenyl ester (8g)
Off-white oil (45.6%): R f = 0.74 (CHCl3); 1H-NMR (300 MHz, CDCl3) δ 7.65 (d, J = 8.03 Hz, 2H), 7.5 (d, J = 7.61 Hz, 2H), 7.32-7.45 (m, 7H), 7.25 (d, J = 8.09 Hz, 2H), 6.75 (dd, J = 9.13, 2.15 Hz, 2H), 6.53 (dd, J = 9.18, 2.16 Hz, 2H), 4.65 (s, 4H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.36 (1C), 145.22 (1C), 141.17 (1C), 138.85 (2C), 132.40 (1C), 131.42 (1C), 130.00 (2C), 129.66 (2C), 129.37 (2C), 128.61 (2C), 124.32 (1C), 124.27 (2C), 124.22 (2C), 124.17 (1C), 123.36 (1C), 123.23 (1C), 122.23 (1C), 113.20 (2C), 54.50 (2C), 21.74 ppm (1C); IR (thin film) cm−1 3051, 2963, 1601, 1512, 1451; MS (ESI, positive mode) m/z [M + H]+ 580.13669 (C29H24F6NO3S requires 580.13028).
Toluene-4-sulfonic acid 4-[bis-(3-chloro-benzyl)-amino]-phenyl ester (8h)
White oil (14.6%): R f = 0.68 (CHCl3); 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.33 Hz, 2H), 7.2-7.32 (m, 6H), 7.15 (s, 2H), 7.05 (m, 2H), 6.75 (dd, J = 9.21, 2.28 Hz, 2H), 6.51 (dd, J = 9.23, 2.29 Hz, 2H), 4.55 (s, 4H), 2.43 ppm (s, 3 H); 13C-NMR (300 MHz, CDCl3) δ 147.37 (1C), 145.13 (1C), 140.98 (1C), 140.05 (2C), 134.79 (2C), 132.60 (1C), 130.15 (2C), 129.67 (2C), 128.60 (2C), 127.49 (2C), 126.65 (2C), 124.78 (2C), 123.29 (2C), 113.04 (2C), 54.28 (2C), 21.75 ppm (1C); IR (thin film) cm−1 3055, 2963, 1597, 1574, 1508, 1431; MS (ESI, positive mode) m/z [M + Na]+ 534.06679 (C27H23Cl2NNaO3S requires 534.07757).
Toluene-4-sulfonic acid 4-[bis-(4-chloro-benzyl)-amino]-phenyl ester (8i)
White powder (10.4%): R f = 0.70 (CHCl3); mp. 151–152°C; 1H-NMR (300 MHz, CDCl3) δ 7.67 (d, J = 8.26 Hz, 2H), 7.25-7.32 (m, 6H), 7.10 (d, J = 8.31 Hz, 4H), 6.75 (dd, J = 9.07, 1.99 Hz, 2H), 6.51 (dd, J = 9.13, 2.19 Hz, 2H), 4.50 (s, 4H), 2.40 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.56 (1C), 145.08 (1C), 140.93 (1C), 136.29 (1C), 132.98 (2C), 132.83 (2C), 129.64 (2C), 128.93 (4C), 128.55 (2C), 128.06 (4C), 123.21 (2C), 113.22 (2C), 54.15 (2C), 21.72 ppm (1C); IR (thin film) cm−1 3048, 2963, 1601, 1508, 1489, 1443; MS (ESI, positive mode) m/z [M + H]+ 512.08485 (C27H24Cl2NO3S requires 512.07757); Anal. Calcd for C27H23Cl2NO3S: C 63.28, H 4.52, Cl 13.84, N 2.73, S 6.26, found: C 62.19, H 4.92, Cl 13.56, N 2.51, S 6.88.
Toluene-4-sulfonic acid 4-[bis-(3-methyl-benzyl)-amino]-phenyl ester (8j)
Off-white powder (6.4%): R f = 0.68 (CHCl3); mp. 90–91°C; 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.28 Hz, 2H), 7.18–7.32 (m, 6H), 7.08 (d, J = 7.52 Hz, 2H), 6.95 (d, J = 6.29 Hz, 4H), 6.70 (dd, J = 9.17, 2.11 Hz, 2H), 6.52 (dd, J = 9.2, 2.16 Hz, 2H), 4.52 (s, 4H), 2.42 (s, 3H), 2.3 ppm (s, 6H); 13C-NMR (300 MHz, CDCl3) δ 148.12 (1C), 145.01 (1C), 140.27 (1C), 138.46 (2C), 138.07(2C), 132.77 (1C), 129.64 (1C), 128.66 (3C), 128.60 (2C), 127.84 (2C), 127.20 (2C), 123.59 (2C), 123.03 (2C), 112.59 (2C), 54.44 (2C), 21.77 (1C), 21.59 ppm (2C); IR (thin film) cm−1 3047, 2963, 1605, 1512, 1447; MS (ESI, positive mode) m/z [M + H]+ 472.19409 (C29H30NO3S requires 472.18681); Anal. Calcd for C29H29NO3S: C 73.86, H 6.20, N 2.97, S 6.80, found: C 73.73, H 6.60, N 2.04, S 6.88.
Toluene-4-sulfonic acid 4-[bis-(3-bromo-benzyl)-amino]-phenyl ester (8k)
Off-white oil (11%): R f = 0.69 (CHCl3); 1H-NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 7.9 Hz, 2H), 7.38 (dd, J = 14.44, 8.76 Hz, 4H), 7.18 (d, J = 7.77 Hz, 2H), 7.08 (d, J = 7.83 Hz, 2H), 6.74 (d, J = 9.16 Hz, 2H), 6.50 (d, J = 9.20 Hz, 2H), 4.54 (s, 4H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 147.02 (1C), 146.53 (1C), 145.42 (2C), 140.30 (2C), 130.43 (2C), 129.68 (2C), 129.56 (2C), 128.61 (4C), 125.22 (4C), 123.32 (2C), 113.00 (2C), 54.19 (2C), 29.75 ppm (1C); IR (thin film) cm−1 3048, 2963, 1597, 1508, 1465; MS (ESI, positive mode) m/z [M + Na]+ 624.96507 (C27H23Br2NNaO3S requires 624.35046).
Toluene-4-sulfonic acid 4-[bis-(3-nitro-benzyl)-amino]-phenyl ester (8l)
Yellow powder (17.6%): R f = 0.64 (CHCl3); mp. 133–134°C; 1H-NMR (300 MHz, CDCl3) δ 8.13 (m, 2H), 8.02 (s, 2H), 7.65 (d, J = 8.3 Hz, 2H), 7.5 (m, 4H), 7.25 (d, J = 8.14 Hz, 2H), 6.78 (dd, J = 9.23, 2.12 Hz, 2H), 6.5A3 (dd, J = 9.2, 2.13 Hz, 2H), 4.75 (s, 4H), 2.42 ppm (s, 3H); 13C-NMR (300 MHz, CDCl3) δ 148.77 (1C), 146.77 (1C), 145.28 (1C), 145.20 (1C), 141.60 (1C), 141.58 (1C), 140.02 (2C), 132.75 (2C), 132.41 (1C), 129.99 (2C), 129.72 (2C), 128.57 (2C), 123.59 (2C), 122.60 (2C), 121.58 (2C), 113.50 (2C), 54.57 (2C), 21.75 ppm (1C); IR (thin film) cm−1 3074, 2928, 1603, 1530, 1506, 1350; MS (ESI, positive mode) m/z [M + H]+ 534.13044 (C27H24N3O7S requires 534.12567); Anal. Calcd for C27H23N3O7S: C 60.78, H 4.34, N 7.88, S 6.01, found: C 60.33, H 4.56, N 7.76, S 6.39.
CETP inhibition assay
CETP inhibitory bioactivities were assayed by fluorescent-CE transfer employing commercially available kit (BioVision, Linda Vista Avenue, USA). The assay kit is based on donor molecule containing fluorescent self-quenched neutral lipid that is transferred to an acceptor molecule in the presence of CETP (from rabbit serum). CETP-mediated transfer of the fluorescent neutral lipid to the acceptor molecule results in increase in fluorescence. Inhibition of CETP will prevent lipid transfer and therefore decrease fluorescence intensity.
The assay procedure can be described briefly as follows. An aliquot of 1.5 μl of rabbit serum (CETP) was added to 160 μl of testing sample (12.8 μM). Then, 20 μl of the master mix, provided in the assay kit (5 μl donor molecule, 5 μl acceptor molecule and 10 μl assay buffer), was added, mixed well, and the volume was made up to 203 μl with the provided assay buffer. The assay is carried out using a black 96-well plate with led. After incubation at 37°C for 1 h, fluorescence intensity (Excitation λ: 465 nm; Emission λ: 535 nm) was read in a FLX800TBI Microplate Fluorimeter (BioTek Instruments, Winooski, USA).
The tested compounds were initially dissolved in DMSO to yield 10 mM stock solutions and subsequently diluted to the required concentrations using distilled deionized water (12.8 μM). The final concentration of DMSO was adjusted to 0.1%. The percentage of residual activity of CETP was determined for each compound by comparing the activity of CETP in the presence and absence of the tested compound. Positive controls were tested to assess the degree of CETP inhibition by 0.1% DMSO. CETP was not affected by DMSO. The increase in fluorescence intensity with CETP (rabitt serum) is usually 1.5- to 2-fold over 0.1% DMSO. Negative controls lacking rabbit serum were used as background. All measurements were conducted in duplicates.
Results and discussion
Chemistry
In the current work, the intermediates N-(4-hydroxyphenyl)-4-methylbenzenesulfonamide 4a, N-(4-hydroxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamide 4b, and 4-aminophenyl-4-toluene-sulfonic acid ester 7 were prepared from the reaction of tosyl chloride 2 with p-aminophenol 3 in dichloromethane at room temperature and in the presence of triethylamine, as illustrated in Schemes 1 and 2.
Afterward, the resulting intermediates (4a, 4b, or 7) were reacted with different substituted benzyl bromides 5a–5k in DMF at room temperature and in the presence of sodium hydroxide to prepare the final N-(4-benzyloxyphenyl)-4-methyl-benzenesulfonamides (6a–6g) and N-(4-benzyloxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamides (6h–6l). On the other hand, these intermediates were reacted with substituted benzyl bromides in DMF at room temperature to prepare N-(4-benzylaminophenyl)-toluene-4-sulfonic acid esters (8a–8c) and 4-(N,N)-[bis-(benzylaminophenyl)]-toluene-4-sulfonic acid esters (8d–8l). The highest yield was obtained upon reacting N-(4-hydroxy-phenyl)-4-methyl-benzenesulfonamide with 4-bromo-benzylbromide to give 6b in 63% yield.
Scheme 1 shows the new benzenesulfonamide derivatives (6a–6l), while Scheme 2 shows sulfonic acid ester derivatives (8a–8l).
Biological evaluation
The results of CETP inhibition assay, presented in Tables 1 and 2, demonstrate that compound 6k exhibit appreciable activity against CETP with an IC50 value of 3.4 μM. Although our newly synthesized compounds are of lower potency than some published CETP inhibitors, these derivatives are characterized by their novel scaffold that can serve as a promising leads for further optimization. Tables 1 and 2 show the fit values of the synthesized compounds against Hypo4/8.
Figure 2 shows how Hypo4/8 maps the most active synthesized compounds 6h–6l. The hydrogen bond acceptor feature of Hypo4/8 fits either the sulfonamide moiety or the benzyloxy oxygen, while ring aromatic feature maps one of the aromatic rings in the structure. Furthermore, the three hydrophobic features of Hypo4/8 fit different substituted aromatic rings, i.e., substituted with methyl, trifluoromethyl, chloro, or methoxy.
The mapping of Hypo4/8 against the synthesized compounds 6h (IC50 = 11.1 μM), 6i (IC50 = 10.5 μM), 6j (IC50 = 13.2 μM), 6k (IC50 = 3.4 μM), and 6l (IC50 = 3.9 μM): a Hypo4/8, b structure of 6h, c Hypo4/8 mapped against 6h, d structure of 6i, e Hypo4/8 mapped against 6i, f structure of 6j, g Hypo4/8 mapped against 6j, h structure of 6k, i Hypo4/8 mapped against 6k, j structure of 6l and k Hypo4/8 mapped against 6l
The new compounds 6a–6l and 8a–8l were synthesized to explore the influence of bulkiness of the structure and electronic properties of the substituent (i.e., electron donating or withdrawing group) on the CETP inhibitory activity.
As a general trend, the CETP inhibitory activity for this series increases as the lipophilic character of the compound is enhanced, as can be seen in compounds having four aromatic rings, this is in accordance with the lipophilic binding pocket of CETP (Qiu et al., 2007). Furthermore, the presence of donating groups on the benzyl ring, i.e., methyl and methoxy substituents, seems to contribute positively to the CETP inhibitory activity of the compound, as can be observed in compounds 6k and 6l, while presence of deactivating groups decreases the activity as seen in 6h–6j.
Moreover, from the benzene sulfonamide derivatives 6a–6l, compounds with two sulfone moieties have the best CETP inhibitory activities. These sulfone groups may be involved in significant interactions with the CETP pocket, as well as the size of the sulfur atom contributes positively to the lipophilic character of the compound. Compounds 6a–6l and 8a–8l were tested against CETP at 10 μM concentrations and exhibited CETP inhibitory activity up to 67%. In addition, the CETP IC50 values were determined for the most active synthesized compounds, 6h–6l, 8a, 8h, and 8l, where compound 6k displayed the best activity with an IC50 value of 3.4 μM.
Conclusions
In conclusion, we have successfully accomplished synthetic investigation of a new series of N-(4-benzyloxyphenyl)-4-methyl-benzenesulfonamides, N-(4-benzyloxyphenyl)-N-(4-methylbenzenesulfonyl)-4-methylbenzenesulfonamides, N-(4-benzylaminophenyl)-toluene-4-sulfonic acid esters, and 4-(N,N)-[bis-(benzylaminophenyl)]-toluene-4-sulfonic acid esters as potential CETP inhibitors. Future optimization of the hydrophobic characters together with the substituent’s electronic properties can lead to the discovery of more potent derivatives.
References
Abu Khalaf R, Abu Sheikha G, Bustanji Y, Taha MO (2010) Discovery of new cholesteryl ester transfer protein inhibitors via ligand-based pharmacophore modeling and QSAR analysis followed by synthetic exploration. Eur J Med Chem 45:1598–1617
Abu Sheikha G, Abu Khalaf R, Melhem A, Albadawi G (2010) Design, synthesis, and biological evaluation of benzylamino-methanone based cholesteryl ester transfer protein inhibitors. Molecules 15:5721–5733
Akamatsu M (2002) Current state and perspectives of 3D-QSAR. Curr Top Med Chem 12:1381–1394
Boekholdt SM, Kuivenhoven JA, Wareham NJ et al (2004) Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women. Circulation 110:1418–1423
Castilho MS, Marcelo S, Guido RafaelVC, Andricopulo, Adriano D (2007) 2D Quantitative structure–activity relationship studies on a series of cholesteryl ester transfer protein inhibitors. Bioorg Med Chem 15:6242–6252
Chapman MJ, Le Goff W, Guerin M, Kontush A (2010) Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J 31:149–164
Cronin MTD, Schultz TW (2003) Pitfalls in QSAR. J Mol Struct 622:39–51
Cuchel M, Lund-Katz S, de la Llera-Moya M, Millar JS, Chang D, Fuki I, Rothblat GH, Phillips MC, Rader DJ (2010) Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler Thromb Vasc Biol 30:526–532
Grass DS, Saini U, Felkner RH et al (1995) Transgenic mice expressing both human apolipoprotein B and human CETP have a lipoproyein cholesterol distribution similar to that of normolipidemic humans. J Lipid Res 36:1082–1091
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Hanumantharao P, Sambasivarao SV, Soni LK, Gupta AK, Kaskhedikar SG (2005) QSAR analysis of analogs of bis[2-(acylamino) Ph] disulfides, 2-(acylamino) benzenethiols and S-[2-(acylamino) Ph] alkanethioates as antihyperlipidemic agents. Indian J Chem 44B:1481–1486
Julve J, Llaverias G, Blanco-Vaca F, Escolà-Gil JC (2011) Seeking novel targets for improving in vivo macrophage-specific reverse cholesterol transport: translating basic science into new therapies for the prevention and treatment of atherosclerosis. Curr Vasc Pharmacol 9:220–237
Kelkar MA, Pednekar DV, Pimple SR, Akamanchi KG (2004) 3D QSAR studies of inhibitors of cholesterol ester transfer protein (CETP) by CoMFA, CoMSIA and GFA methodologies. Med Chem Res 13:590–604
Lamarche B, Despres JP, Moorjani S, Cantin B, Dagenais GR, Lupien PJ (1996) Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease, Results from the Quebec cardiovascular study. Atherosclerosis 119:235–245
Lewis GF (2006) Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr Opin Cardiol 21:345–352
McPherson R, Mann CJ, Tall AR, Hogue M, Martin L, Milne RW, Marcel YL (1991) Plasma concentration of cholesteryl ester transfer protein in hyperlipoproteinemia. Arterioscler Thromb 11:797–804
Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D (1990) Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis 10:316–323
Qiu X, Mistry A, Ammirati MJ, Chrunyk BA et al (2007) Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol 14:106–113
Tall AR, Wang N, Mucksavage P (2001) Is it time to modify the reverse cholesterol transport model? J Clin Invest 108:1273–1275
Vasan RS, Pencina MJ, Robins SJ, Zachariah JP, Kaur G, D’Agostino RB, Ordovas JM (2009) Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation 120:2414–2420
Acknowledgments
The authors are grateful to the Scientific Research and Postgraduate Deanship at Al-Zaytoonah University of Jordan for sponsoring this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu Khalaf, R., Abu Sheikha, G., Al-Sha’er, M. et al. Design, synthesis, and biological evaluation of sulfonic acid ester and benzenesulfonamide derivatives as potential CETP inhibitors. Med Chem Res 21, 3669–3680 (2012). https://doi.org/10.1007/s00044-011-9917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9917-5