Abstract
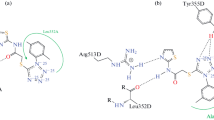
A set of imidazo[1,2-a]pyridine derivatives was submitted to a docking analysis on COX-1 and COX-2. Although most of the compounds showed affinity for both COX-1 and COX-2, compound 1h showed a higher COX-1 affinity while 1i was more selective to COX-2. None of them had ∆G value higher than indomethacin. Compounds 1b, 1c, and 1g were synthesized and evaluated as potential anti-inflammatory agents. Compound 2-(N-cyclopropyl)amino-3-nitroimidazo[1,2-a]pyridine (1c) which gave a very poor affinity for either COX-1 or COX-2, showed anti-inflammatory activity (20 mg/kg) on the cotton pellet granuloma bioassay, with no induction of gastrointestinal damage.






Similar content being viewed by others
References
Akaho E, Fujikawa C, Runion HI, Hill CR, Nakano H (1999) A study on binding modes of nonsteroidal anti-inflammatory drugs to COX-1 and COX-2 as obtain by Dock4.0. J Chem Soft 5(3):147–162
Almirante L, Mugnaini A, Rugarli P, Gamba A, Zefelippo E, De Toma N, Murmann W (1968) Derivatives IF imidazole. III. Synthesis and pharmacological activities of nitriles, amides, and carboxylic acid derivatives of imidazo[1, 2-a]pyridine. J Med Chem 12:122–126
Arias L, Salgado-Zamora H, Cervantes H, Campos E, Reyes A, Taylos E (2006) Some nucleophilic substitutions in 2-cyano-3-nitroimidazol [1,2-α] pyridine. J Heteroc Chem 43(3):565–569
Banchero P, Giachetto G, Telechea H, Speranza N, Seade C (2004) Seguridad de los inhibidores selectivos de la ciclooxigenasa 2. Rev Med Urug 20:136–144
Biftu T, Feng D, Fisher M, Liang G, Qian X, Scribner A, Dennis R, Lee S, Liberator PA, Brown C, Gurnett A, Leavitt PS, Thompson D, Mathew J, Misura A, Samaras S, Tamas T, Sina JF, McNulty KA, McKnight CG, Schmatz DM, Wyvratt M (2006) Synthesis and SAR studies of very potent imidazopyridine antiprotozoal agents. Bioorg Med Chem 16:2479–2483
C-Basurto J, Aburtos J, T-Ferrara J, Torres E (2007) Ligand recognition by chloroperoxidase using molecular interaction fields and quantum chemistry calculations. Mol Simul 33(8):649–654
Chandrasekharan NV, Simmons DL (2004) The cyclooxygenases. Genome Biol 5:241.1–241.7
Davies NM, Good RL, Roupe KA, Yáñez JA (2004) Cyclooxygenase-3: axiom, dogma, anomaly, enigma or splice error?—not as easy as 1, 2, 3. J Pharm Sci 7(2):217–226
Dilber SP, Dobric SL, Juranic ZD, Markovic BD, Vladimirov SM, Juranic IO (2008) Docking studies and anti-inflammatory activity of β-hidroxy-β-arylpropanoic acids. MOLEFW 13:603–615
Felcy FG, Lakshminarasimhan D, Vasantha P, Kuppuswamy N (2001) Cyclooxygenase-2-an attractive target for fruitful drug design. Curr Sci 80(1):26–34
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Peterson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.9. Gaussian, Inc., Pittsburgh
Hayakawa M, Kaizawa H, Kawaguchi K, Ishikawa N, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S, Raynaud FI, Waterfield MD, Parker P, Workman P (2007) Synthesis and biological evaluation of imidazo[1, 2-α]pyridine derivates as novel PI3 kinase p110α inhibitors. Bioorg Med Chem 15:403–412
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Kaapro A, Ojanen J (2002) Protein docking. http://www.lce.hut.fi/teaching/S-114.500/k2002/Protdock.pdf. Accessed 5 Oct 2009
Kaminski JJ, Doweyko AM (1997) Antiulcer agents 6. Analysis of the in vitro biochemical and in vivo gastric antisecretoru activity of substituted imidazo[1, 2-a]pyridines and related analogues using comparative molecular field analysis and hypothetical active site lattice methodologies. J Med Chem 40:427–436
Kumar V, Abbas AK, Fausto N (2005) Patología estructural y funcional, 7th edn edn. Elsevier, España, pp 48–117
Kunanusorn P, Teekachunhatean S, Sangdee C, Panthong A (2009) Antinociceptive and anti-inflammatory activities of a Chinese herbal recipe (DJW) in animal models. Int J Appl Res Nat Prod 2(1):1–8
Lacerda RB, de Lima CKF, da Silva LL, Romeiro NC, Miranda ALP, Barreiro EJ, Fraga CAM (2009) Discovery of novel analgesic and anti-inflammatory 3-arylamine-imidazo[1, 2-α]pyridine symbiotic prototypes. Bioorg Med Chem 17:74–84
Lhassani M, Chavignon O, Chezal J, Teulade J, Chapat J, Snoeck R, Andre G, Balzarini J, de Clercq E, Gueiffier A (1999) Synthesis and antiviral activity of imidazo[1, 2-a]pyridines. Eur J Med Chem 34:271–274
Márquez-Flores YK, Montellano-Rosales H, Campos-Aldrete ME, Meléndez-Camargo ME (2009) Anti-inflammatory activity of aqueous and methanolic extracts of Oenothera rosea L′Hér. ex Ait in the rat. Rev Mex Cien Farm 40(3):11–16
Maruyama Y, Anami K, Terasawa M, Goto K, Imayoshi T, Kadobe Y, Mizushima Y (1981) Anti-inflammatory activity of an imidazopyridine derivative (miroprofen). Arzneim Forsch 31(7):1111–1118
MirAfzali Z, Leipprandt JR, McCracken JL, DeWitt DL (2006) Topography of the prostaglandin endoperoxide H2 synthase-2 in membranes. J Biol Chem 281(38):28354–28364
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639
Padilla ZAJ, Rojo DA (2002) Simulación del reconocimiento entre proteínas y moléculas orgánicas o docking. Aplicación al diseño de fármacos. Mensaje Bioquím 26:129–145
PDB. Protein Data Bank. http://www.pdb.org/pdb/home/home.do. Accessed 9 Sept 2009
Penning TD, Chandrakumar NS, Desai BN, Djuric SW, Gasiecki AF, Malecha JW, Miyashiro JM, Russell MA, Askonas LJ, Gierse JK, Harding EI, Highkin MK, Kachur JF, Kim SH, Villani-Price D, Pyla EY, Ghoreishi-Haack NS, Smith WG (2003) Synthesis of imidazopyridines and purines as potent inhibitors of leukotriene A4 hydrolase. Bioorg Med Chem Lett 13:1137–1139
Pradilla VOE (2004) Ciclooxigenasa 3: La nueva iso-enzima en la familia. MedUNAM 7(21):181–184
Rajeswari R, Thejomoorthy P, Mathuram LN, Narayana RKVS (2006) Anti-inflammatory activity of Cassia Fistula Linn. Bark extracts in sub-acute models of inflammation in rats. Vet Anim Sci 2(5):193–199
Rether J, Erkel G, Anke T, Bajtner J, Sterner O (2008) Imidazo[1, 2-a]pyridine derivates as inhibitors of TNF-α expression in T cells. Bioorg Med Chem 16:1236–1241
Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (1999) Technical specifications for the production, care and use of laboratory animals. Official Mexican Standard. NOM-062.ZOO
Teulade JC, Grassy G, Girard JP, Chapat JP, De Buoochberg MS (1978) Antibactériens dérives de la nitro-3 imidazo [1,2-a] pyridine. Synthése et relation structure-activité “in vitro”. Eur J Med Chem 13(3):271–276
Thejomoorthy P, Narayana RKVS (2007) Studies on anti inflammatory and analgesic activity of Seendil Chooranam—a Siddha drug. Vet Anim Sci 3(2):78–82
Acknowledgment
This study was partially supported by the Secretaría de Investigación y de Posgrado, COFAA, (IPN). ICyTDF and CONACYT Grant No. 49937, 62488, 91426.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Márquez-Flores, Y.K., Campos-Aldrete, M.E., Salgado-Zamora, H. et al. Docking simulations, synthesis, and anti-inflammatory activity evaluation of 2-(N-alkyl)amino-3-nitroimidazo[1,2-a]pyridines. Med Chem Res 21, 775–782 (2012). https://doi.org/10.1007/s00044-011-9585-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9585-5