Abstract
An intriguing yet little studied aspect of social insect foraging is the use of resources other than food. We are interested in the collection of plant resins for nest construction and defense by tropical stingless bees. However, direct observations of stingless bee foraging and potential predation activities by natural enemies at resin sources are particularly rare and therefore require a trade-off between observation time and the number of sources observed. We used affordable remote microcontroller-based camera traps to enable for longer durations of continuous and simultaneous monitoring of resin foraging at multiple resin sources in an undisturbed lowland dipterocarp rainforest in Brunei Darussalam (Borneo). Analysis of photos from camera traps shows that stingless bee visitation to resin sources was uncommon at resin wounds in the forest understory (27.3%). Bees visiting wounds displayed a propensity for short and regular resin foraging bouts of up to a few days to particular resin sources. Where there were encounters between stingless bees and natural enemies, i.e., assassin bugs at resin sources, there was a 100% predation success rate (n = 4). Our study suggests that microcontroller-based camera traps complement or may even replace in-person field observations, in particular for observations of organisms or interactions occurring at low abundance. They allow for systemically collected observations which can form the basis for hypothesis-driven research as part of “next-generation natural history”.
Similar content being viewed by others
Introduction
The use of non-floral resources such as resin by bees for nest construction is well documented for both social and non-social bees (Shanahan and Spivak 2021; Chui et al. 2021). Stingless bees (Apidae: Meliponini) are pantropical highly eusocial insects that collect tree resin, among other materials such as mud and fecal matter, for use in nest construction, defense, communication, and for its anti-microbial properties (Michener 1961; Roubik 1989, 2006; Grüter 2021; refer to review by Shanahan and Spivak 2021 for details on the uses and storage of resin within the nest environment). Major sources of tree resin for stingless bees include members of plant families such as the Dipterocarpaceae in Southeast Asia, the Fabaceae in tropical Africa and America, and pantropical Burseraceae (Langenheim 2003). Resin is usually produced by trees on trunk/branch or cut surfaces as a response to injury, either through mechanical (e.g., branch breakage) or biological (e.g., burrowing beetle larvae) (Langenheim 2003) means, though occasional exudations on the bark surface of some dipterocarp species can also occur (Ashton 1964, 1968; Meijer and Wood 1964; Symington 2004).
Little is known of the precise sources of resin of Southeast Asian stingless bees. A Bornean study found that stingless bees had a general preference for dipterocarp resin, but did not necessarily visit trees which produced “copious amounts” of resin, even when they were adjacent to individuals visited for their small amounts of resin (Leonhardt and Blüthgen 2009). Stingless bees are able to differentiate resin from individual trees, which suggests that resin foragers are able to learn the specific resin chemical profiles of tree individuals (Leonhardt et al. 2010). However, the mechanisms that underlie stingless bee resin foraging preferences and can explain their decisions in context remain unknown.
While there have been many studies that examined the activity pattern of resource collection, including resin, via returning stingless bee foragers at nest entrances (Inoue et al. 1985; Biesmeijer 1997; de Bruijn and Sommeijer 1997; Mohammed and Starr 1999; Leonhardt et al. 2007; Barbosa et al. 2016), far fewer studies have looked at the foraging activity of stingless bees at resin sources. Seminal papers by Johnson (1983) and Howard (1985) described the competitive and aggressive interspecific interactions of Costa Rican stingless bees at resin sources, which were later also reported by Leonhardt and Blüthgen (2009) in Bornean stingless bees. Moreover, little has been documented of the interactions between stingless bees and their predators at resin sources in tropical rainforests. There are a few descriptive field observations of predation of stingless bees by resin assassin bugs (Reduviidae: Harpactorinae: Apiomerini, Ectinoderini, and Diaspidini). Resin assassin bugs are known to lie in wait near resin sources to ambush their prey (Roepke 1932; Usinger 1958; Janzen 1985). Resin assassin bugs “dip the[ir] front legs in sticky exudations to cover the tibiae and one segmented clawless tarsi. … The legs are held forward like those of a pseudoscorpion and are used to trap the bees.” (Usinger 1958). It would appear that resin assassin bugs ambush their prey with front claws coated with a sticky substance, e.g., resin, and hold these sticky implements ahead of them to ensnare any prey encountered. However, due to the low frequency of such encounters, the hunting strategies and occurrence of assassin bugs at resin sources, and their potential impact on stingless bees are as yet hardly known.
Direct observations of stingless bee foraging activities (but also of other bee species) at resin sources require a trade-off between observation time and the number of sources observed. Past studies conducted either long-time observations (30 h over 5 days as in Howard 1985) at a single resin source, or several short-time observations (3–15 min as in Leonhardt and Blüthgen 2009) at multiple resin sources. Neither approach will enable simultaneous continuous multi-day observations of bees at multiple resin sources within a sampled plot. Against a backdrop of a decline in ecological field research (Ríos-Saldaña et al. 2018), recent advances in sensor and recording technology, such as for camera trapping, are revitalizing field biology in what is termed “next-generation natural history”, with systemically collected observations that can form the basis for hypothesis-driven research (Tosa et al. 2021).
In this study, we tested if small, affordable, remote camera traps can enable longer durations of continuous and simultaneous monitoring of resin foraging at multiple resin sources in the understory of a remote primary tropical rainforest and may thus complement or even replace in-person field observations. Typically, off-the-shelf solutions for non-obstructive remote observations and detection of wildlife involve the use of bulky, heavy, and costly (< 250–1000 USD per unit) camera traps (Rovero et al. 2013). These camera traps use PIR (Passive Infrared) sensors to detect motion in endotherms, which precludes its effective use on ectotherms such as insects. Instead, an LIDAR (light radar) motion trigger system can be used in tandem with a DSLR (digital single lens reflex) camera and external flashes (Sabre by Cognisys Systems, Inc.) to capture images of insects, as shown for hawk moth visitation to a ghost orchid (Danaher et al. 2019). Such camera traps to detect and photograph endotherms are, however, very expensive when used for studies that require many simultaneous observations (Droissart et al. 2021).
In recent years, the adoption of Single Board Computers (SBC), such as the popular Raspberry Pi platform in the biological sciences, has been a segue for further understanding interactions between organisms and their natural environment (Jolles 2021). In particular, the PICT, a relatively low-cost camera trap, recently developed around the Raspberry Pi Zero that could remotely record up to 72 h of video of insect–flower interactions in the tropical field environment, has further pushed the envelope of realizing more affordable applications of camera trapping (Droissart et al. 2021). A substantial battery (30,000 mAh–111 Wh) is recommended for use with the power requirements of PICT (0.76 W during day, 1.87 W at night), which allows for either 72 h of continuous video capture, or up to 9 days of daytime-only video capture (Table 1) (Droissart et al. 2021). However, costs for a single PICT unit are ~ < 170 USD which renders usage of multiple units also very expensive.
The presented study as well as many other studies require a repetitive series of tasks on a battery-operated system (elaborated below). Here, microcontrollers, such as the Raspberry Pi Pico, Arduino, or ESP32, may be sufficient, as SBCs, while more computationally capable, have higher energy demands during operation and cannot engage in a 'deep sleep' mode to conserve energy without the installation of additional hardware. Remote operation of the camera traps in field conditions under battery power necessitates the efficient use of power (current draw) while in operation and the native capability of microcontrollers to enter a 'deep sleep' mode between tasks.
Summarized, the requirements of a camera trap for capturing images of insect visitors at, e.g., resin sources are as follows: (1) per unit cost effectiveness with commercially available parts for ease of scalability of 40 units or more; (2) close-range operation (< 100 mm) to effectively capture small insects of at least 3 mm body length (i.e., stingless bees, other resin source visitors); (3) operation during daylight hours, independent of lighting conditions, with a wide dynamic range (i.e., deep shadow under closed canopy conditions with sunflecks; insolation of trunk surface); (4) shielding from rain (i.e., canopy throughfall and trunk stemflow) (see Suppl. File S1 for detailed requirements). To meet these requirements, we selected and tested an ESP32-CAM microcontroller-based camera trap with a custom designed and fused deposition modeling (FDM) 3D-printed housing (Fig. 1) and run program (Table 1, Suppl. File S2). Using this small, relatively affordable camera trap system, we investigated the hitherto little studied visitation patterns of stingless bees to dipterocarp resin wounds, and the interactions between resin foraging stingless bees and their predators.
Camera trap parts. a Profile view: (i) OV2640 camera lens, (ii) microSD card, (iii) photo-resistor, (iv) hole for camera flash, (v) camera lens—resin wound alignment markings; b Camera trap interior: (i) ESP32-CAM, (ii) battery caps, and (iii) 18,650 LiFePO4 battery; c Camera trap electronic wiring diagram
Methods
Between 15 and 31 July 2021, dipterocarp trees with a minimum diameter-at-breast-height (DBH) of 30 cm were surveyed for naturally occurring resin wounds in 13 equally distributed 1 × 1 ha sub-plots within a 25 ha Forest Dynamics Plot (Smithsonian Tropical Forest Institute) located in the primary forests of Ulu Temburong National Park, accessed from Kuala Belalong Field Studies Centre, at Kuala Belalong, Brunei Darussalam. Kuala Belalong has particularly wet and humid rainforests. Annual mean temperature ranges from 22.3 to 32.5 °C. Annual mean relative humidity is > 90% with a yearly average of 240 rain-days and typically 4000–5000 mm of rainfall (Dykes 2000). Resin wounds were assessed for suitability in the deployment of camera traps; wounds situated along or between tree buttresses were not considered as the camera trap could not be secured in place. To preclude potential harm to trees monitored (e.g., caused by nails), camera traps were attached to tree trunks using rope and a quick release mounting plate (Fig. 2d). The camera traps were positioned approximately 100 mm from resin wounds at an angle of 45° (Fig. 2), thereby ensuring that the resin wounds were obscured neither visually nor physically from stingless bees and other resin foragers. These camera traps do not emit sound, and only a weak white light is present when the camera is active. We therefore believe that the camera trap set up and light did not deter visitation by stingless bees and other resin foragers as evidenced by the multiple visits made at some wounds with camera traps.
A total of 40 camera traps were on rotational deployment on overall 22 dipterocarp trees with naturally occurring active resin wounds. If a camera trap reported errors during battery replacement, a spare camera trap unit would substitute the defective unit, minimizing coverage downtime. Defective units were repaired and inspected before reintroduction into the pool of deployable camera traps. Camera traps took one photo every 30 s during the day when there was sufficient light, and otherwise remained in the power saving ‘deep sleep’ mode. With a 30 s interval between photo captures, it is possible that the camera traps missed visitations by stingless bees and other resin foragers if the duration of their resin foraging bout lasted for fewer than 30 s. However, prior observations of stingless bee foragers at resin wounds indicate that they tended to spend more than 30 s foraging for resin (Chui SX pers. obs.; Leonhardt SD pers. obs.).
The ESP32-CAM platform comes configured with a microSD card slot and bundled with an OV2640 2MP camera, for an affordable ~ 7 USD / unit (Banggood.com) with low-power consumption (Table 1). Including a 16 GB microSD card and 18,650 LiFePO4 battery, the total cost of a single camera trap unit amounts to 16 USD, in contrast to the 109–169 USD for a PICT (Table 1). Camera traps were expected to run for about 4.42 days (Table 1), and so, each camera trap run was carried out for 4 days. Before the start of each camera run, a formatted microSD card and a charged 18,650 LiFePO4 battery were inserted in the camera trap, in exchange with the data-containing microSD card, and drained battery from the previous run. A total of 4 camera trap runs were carried out for each of the 22 dipterocarp trees with active resin wounds. Photos from the microSD cards were uploaded to a computer, and manually filtered for photos containing any resin wound visitors. As many photos had low contrast or strong changes in lighting and shadows between photos, depending on the time of day, automated comparison of image frames to detect subjects of interest with software (Norouzzadeh et al. 2018; Fennell et al. 2022) was untenable.
From the filtered stack of camera trap photos that contained resin wound visitors, visual identification to species was made for stingless bees (determinations by Chui SX), while probable identifications to genera were made for other organisms (i.e., wasps and assassin bugs) with the help of external expertise [wasps: iNaturalist users brian-d and marco_selis; assassin bugs: Hwang WS (Lee Kong Chian Natural History Museum, Singapore)]. In addition, photos were sorted by category of activity presented (i.e., resin foraging, ambush, and predation). The timing of resin wound visitation was retrieved for each photo, and the duration of visitation events was determined by the difference in start and end timings of consecutive photos of visitors; a break (> 60 s or 2 photos) in the photo sequence from the departure of the visitor was treated as a separator of discrete events.
Data analyses
To provide visualization of data distribution for the duration of stingless bee foraging bouts to resin sources and the interval periods between bouts, as well as for the duration of assassin bug activities at resin sources, bean plots were generated with the beanplot R package (V1.3.1; Kampstra 2008). As a majority of camera trap observations were of small sample sizes, we did not conduct any statistical significance test. Instead, data visualizations and descriptive statistics were performed in RStudio version 1.1.453 (RStudio Team 2016).
Results
Visitation to resin sources
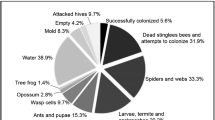
From camera traps deployed at 22 dipterocarp trees with naturally occurring resin sources, 12 (54.5%) detected activity at resin sources, while the remaining 10 did not (Figs. 3-1, -2; refer to Table 2 for tree IDs and species determinations). A total of four stingless bee species (Fig. 4a–d) were recorded at six resin sources; Lepidotrigona ventralis was the most common stingless bee species detected foraging at five resin sources [tree IDs 194318 (Shorea exelliptica): 5 observations; 243475 (Shorea agamii): 25 observations; 253439 (Shorea confusa): 116 observations; 253851 (Shorea argentifolia): 45 observations; 253887 (Vatica odorata ssp. mindanensis): 1 observation; Fig. 3-1a–d, f], Tetragonula melina was detected foraging at two resin sources [tree IDs 253439 (Shorea confusa): 21 observations; 243475 (Shorea agamii): 2 observations; Fig. 3-1a, c], and Tetragonula (Tetragonilla) collina was detected foraging at a single resin source [tree ID 194318 (Shorea exelliptica): 21 observations; Fig. 3-1d], while Tetragonula melanocephala foraged at a resin source just once [tree ID 94239 (Shorea obscura); Fig. 3-1e]. Inter-specific interactions between stingless bee species at resin sources were not observed. While the two stingless bee species L. ventralis and T. melina foraged at the same resin source, there was just one short instance when both species were observed foraging simultaneously Fig. 3-1a: Day 4, 1422H-1424H). Stingless bees were found to forage resin from six of the 22 dipterocarp trees (Fig. 3-1a–f). Besides stingless bees, three wasp species (Fig. 4e–g) were detected foraging at five resin sources (Fig. 3-1a, d; -2g, h, k). The resin potter wasp Epsilon sp. (Vespidae, Eumeninae) was detected foraging at four resin sources [tree IDs 33537 (Shorea confusa): 38 observations; 112747 (Shorea parvifolia ssp. velutinata): 1 observation; 243448 (Dipterocarpus sp.): 1 observation; 253439 (Shorea confusa): 39 observations; Fig. 3-1a; -2g, h, k], an unidentified eumenine (Vespidae, Eumeninae) was detected foraging at two resin sources [tree IDs 194318 (Shorea exelliptica): 14 observations; 243448 (Dipterocarpus sp.): 1 observation; Fig. 3-1d; -2g], and Liris sp. (Crabronidae) was detected at a single resin source [tree ID 243448 (Dipterocarpus sp.): 7 observations; Fig. 3-2g]. Eumenine wasps were found to forage resin from five of the resin sources.
(1) Visitation activity at resin wounds. Columns indicate daily duration camera traps were active, solid color bands resin foraging activity, hollow bands ambush activity, and stars predation instances by Ectinoderus sp. (Reduviidae) on stingless bees. Differently colored solid bands represent different stingless bee species and wasps, or reduviid bug developmental states. Tree IDs with species identifications for trees on which these camera traps were deployed as follows: a 253439: Shorea confusa, b 253851: Shorea argentifolia, c 243475: Shorea agamii, d 194318: Shorea exelliptica, e 94239: Shorea obscura, f 253887: Vatica odorata ssp. mindanensis. (2) Visitation activity at resin wounds. Columns indicate daily duration camera traps were active, solid color bands resin foraging activity, and hollow bands ambush activity. Differently colored solid bands represent different wasp species or reduviid bug developmental states. Tree IDs with species identifications for trees on which these camera traps were deployed as follows: g 243448: Dipterocarpus sp., h 33537: Shorea confusa, i 30733: Shorea macroptera ssp. macropterifolia, j 20489: Hopea bracteata, k 112747: Shorea parvifolia ssp. velutinata, l 101237: Shorea atrinervosa
Dipterocarp tree resin wound visitors trapped with installed cameras. Stingless bees: a Lepidotrigona ventralis, b Tetragonula (Tetragonilla) collina, c Tetragonula melanocephala, d Tetragonula melina; Wasps: e Epsilon sp. (Vespidae, Eumeninae), f unidentified eumenine (Vespidae, Eumeninae), g Liris sp. (Crabronidae); Assassin bugs: h Ectinoderus sp. adult, i Ectinoderus sp. nymph
Collectively, stingless bees in this study foraged for resin from resin sources throughout the day, from 0830H (Fig. 3-1b, 13 August 2021) to 1630H (Fig. 3-1d, 16 August 2021), while wasps tended to forage after noon (Fig. 3-1a, d; -2g, h). Stingless bees made multiple consecutive foraging bouts on days that they did visit a resin source (Fig. 3-1a–d). Individual stingless bee species at each resin source spent a few minutes (x̄: 0.80–4.16 min) foraging on resin sources per foraging bout, with longer intervals between foraging bouts (x̄: 8.61–59.55 min) (Fig. 5). Assassin bugs spent longer periods of time foraging for resin (x̄: adults 9.37 min, nymphs 3.51 min) as compared to stingless bees (Fig. 6). Interestingly, only assassin bug nymphs engaged in ambushing behavior at resin sources (x̄: 14.47 min).
Beanplot of duration of dipterocarp tree resin wound foraging bouts and intervals between foraging bouts of stingless bees (n). Data for foraging bouts in white, intervals between foraging bouts in gray; mean = bold line, data = stacked lines (wider lines indicate more values). Tree IDs with species identifications for trees as follows: 194318: Shorea exelliptica, 243475: Shorea agamii, 253439: Shorea confusa, 253851: Shorea argentifolia
Assassin bug Ectinoderus sp. (Reduviidae) adults and nymphs (Fig. 4h, i) were present at resin sources on seven trees, and either foraged for resin or engaged in ambush activity (Fig. 3-1a, c, e; -2i, j, k, l). Both adults and nymphs foraged for resin at resin sources throughout the day, but started and ended foraging later than stingless bees that foraged from the same resin source (Fig. 3-1a, c). Encounters between stingless bees and assassin bugs were infrequent despite ambushing by nymphs at resin sources (Fig. 7b; Suppl. Fig. S5e-h). Four encounters were recorded in total, which resulted in four predation events by assassin bugs (one adult, three nymphs) (Fig. 7a; Suppl. Fig. S5a-d) at the same resin source (Fig. 3-1a).
Functioning of microcontroller-based camera traps
Camera traps were deployed for four consecutive runs, which spanned a collective total of 19 days (Suppl. Fig. S3). Photo-resistor failure occurred in 26 out of the 40 installed cameras, which resulted in camera traps remaining active even in complete darkness, reducing the total runtime of the camera trap on a single set of batteries from an average of 72.46 ± 22.91 (n = 51) h to an average of 60.51 ± 16.98 (n = 22) hours. Poor contact with the battery, leading to inconsistent voltage delivery to the camera trap, was likely responsible for the failure of additional six camera trap runs which lasted an average of 2.31 ± 2.06 h. The camera trap housing was generally sufficient to protect the ESP32-CAM and battery from rain, although, in one instance, failure of the camera trap occurred when a sizeable falling branch cracked the camera trap cover and removed a corner, which exposed the electronics to rain and shorted the microcontroller (Suppl. Fig. S4). Camera traps continued capturing photos during rain if ambient illumination was sufficient to trigger the photo-resistors. As battery replacements were scheduled after 4 days before the start of the next run, a reduction of runtime in some camera traps led to punctuated or shortened observation windows (Suppl. Fig. S3).
Discussion
Stingless bee visitation to resin sources
In this study, stingless bee resin forager visits to dipterocarp tree resin sources were rare; only 6 of 22 (27.3%) camera traps detected stingless bees, with 4 (18%) that were visited more than once over multiple days. In contrast, Leonhardt and Blüthgen observed stingless bees visiting a higher proportion (54.5%, 12 of 22) of dipterocarp resin sources in primary and secondary forest sites in Borneo (Leonhardt and Blüthgen 2009, Suppl. Table S1). Stingless bee species appeared to return to the same resin source within the day, with the propensity for additional days of foraging fidelity (Fig. 3-1a–d). This pattern of multiple days of resin source visitation was most apparent with Lepidotrigona ventralis, which foraged from the same resin source for 3 (Fig. 3-1b) and 6 consecutive days (Fig. 3-1a), and with Tetragonula (Tetragonilla) collina, which foraged from the same resin source for 3 consecutive days (Fig. 3-1d). In contrast, L. ventralis (Fig. 3-1c) and Tetragonula melina (Fig. 3-1a) each foraged at their respective resin sources for just a single day. Curiously, L. ventralis (Fig. 3-1f) and Tetragonula melanocephala (Fig. 3-1e) visited their respective resin sources just once too. While it is known that stingless bees use olfactory cues to locate resin sources and are able to learn and differentiate tree species and possibly even individuals (Leonhardt et al. 2010), much remains to be understood of the individual decision-making of stingless bee resin foragers, in particular decisions pertaining to which resin sources are utilized, when they are visited, and when they are eventually abandoned.
Visitation patterns
Johnson and Hubbell (1974) and Howard (1985) postulated that the observed aggression of Neotropical stingless bee species around resin sources was due to the defensibility of scarce concentrated resources worth defending. While Leonhardt and Blüthgen (2009) observed similar aggressive defending for resin wounds on 10 of 35 trees in a Paleotropical forest, they noted that the greater number of available resin sources, likely due to the dominance of dipterocarp trees in Borneo, could explain the comparatively lower incidences of resin source defending by stingless bees. In this study, we did not record any indications of stingless bee aggregated resin foraging behavior or an aggression hierarchy as previously reported (Johnson 1983; Howard 1985; Leonhardt and Blüthgen 2009). This finding is likely a consequence of the non-overlap or sparseness of foraging bouts to resin sources in the primary forest studied (Fig. 3-1). In fact, almost all foraging bouts had single stingless bee foragers, with a single bout including an individual each of Lepidotrigona ventralis and Tetragonula melina (Fig. 3-1a: Day 4, 1422H-1424H). The lack of multiple resin foragers at resin sources, together with the dispersed and common availability of resin sources in this forest plot (Chui et al., unpubl. data), which concurs with Leonhardt and Blüthgen (2009), suggests that resin availability is possibly not constrained for the population density of bees present in the primary rainforest habitat studied.
Individual stingless bee resin foraging bouts were short (x̄: 0.80–4.16 min) and repetitive (Fig. 5). The bees were observed not leaving the surface of the resin source during the foraging bout, during which resin was collected with their mandibles and transferred to the corbiculae. The duration between foraging bouts (x̄: 8.61–59.55 min) for each stingless bee species at each resin source might be related to the distance of the resin source from the respective stingless bee nests (Fig. 5). Resin sources farther from the nest are expected to be visited at a lower frequency and with longer time intervals between visits than nearer resin sources. As the stingless bees photographed by the camera traps were not marked, it cannot be determined if the resin foragers observed were the same individuals or several different individuals.
Predation at resin wounds
We observed only four encounters between assassin bugs Ectinoderus sp. and stingless bees (Fig. 3-1a). The majority of resin sources that assassin bugs were recorded at were not visited by stingless bees. Moreover, stingless bees and assassin bugs collected resin at different times of the day. Assassin bugs tended to start collecting resin earlier in the morning and later in the afternoon than stingless bees (Fig. 3-1a, c). Once sufficient fresh resin had been collected to coat their front tarsal claws, the assassin bug typically left the resin source, presumably to hunt prey with their sticky coated claws (Roepke 1932; Usinger 1958; Janzen 1985), and returned to the resin source only to re-coat their claws with a fresh layer of resin. Foraging resin and re-coating their claws took longer than foraging bouts of stingless bees (Fig. 6, x̄: adults 9.37 min, nymphs 3.51 min). Stingless bees started foraging for resin later in the morning, but foraged for resin throughout the day and stopped earlier in the afternoon (Fig. 3-1a–d).
At the one resin source where there were multiple encounters between stingless bees and assassin bugs, there appeared to be fewer stingless bee resin foraging bouts with each successful predation event (Fig. 3-1a). Coupled with the mostly singular occurrence of stingless bees at resin sources, predation by assassin bugs may have depleted the group of stingless bee resin foragers exploiting this specific resin source. If predation success were to be measured by encounters between assassin bugs and stingless bees, the success rate would be a 100%, with all observed encounters (n = 4) leading to the capture of the stingless bee. Resin foraging stingless bees, which are typically static on a relatively two-dimensional interface for minutes during each foraging bout, thus appear to present an easy target for an ambush predator. However, the observed rarity of encounters of stingless bees at resin sources in primary forests (Chui SX pers. obs.; Leonhardt SD pers. obs.) hints at the scarcity of stingless bees in the understory of intact forests, and thus the availability of predation opportunities for assassin bugs at resin sources. It would be interesting to test if assassin bugs spent more time ambushing stingless bees at resin sources at places where visitation frequency of resin wounds by stingless bees appears to be higher, as seems to be the case for more disturbed rainforest habitats in the Paleotropics or in Neotropical forests (Howard 1985; Leonhardt and Blüthgen 2009).
Interestingly, three of four predation events were by assassin bug nymphs (Fig. 3-1a). Periods where assassin bug nymphs engaged in ambushing behavior coincided with stingless bee foraging bouts, which led to additional encounters and hence predation events, suggesting that nymphs are able to ‘eavesdrop’ on stingless bee resin foraging behavior. It appears, however, that resin sources are not the primary hunting area for assassin bug adults in our study area, as adults never engaged in ambushing behavior at resin sources (Fig. 6). Unlike adult assassin bugs that seem to solely utilize resin sources as a station to replenish the resin coat of their claws, assassin bug nymphs frequently engaged in ambush behavior at the resin source between resin coatings of their claws. In two instances, nymphs were even found to frequent excavated shallow chambers within older, more viscous resin masses, staying within these chambers for periods a time (Suppl. Fig. S5e–h). As such, assassin bug nymphs may depend on resin sources for both source of material for hunting and hunting ground, as compared to adults that appear to hunt farther afield.
Other resin source visitors
Apart from stingless bees and assassin bugs, the only other organisms that visited resin sources were two eumenine wasps, the resin potter wasp Epsilon sp. and an unidentified eumenine, and a crabronid wasp Liris sp. While Epsilon sp. are known to construct resin nests and are thus in need of resin as a nest building material, the nesting biology of the unidentified eumenine is unknown. Its foraging resin from the resin source indicates the possible use of resin in nesting or nest defense. In the case of Liris sp., detailed analysis of camera trap photos revealed that the wasp spent time at the resin source, but did not collect any resin, suggesting that its presence was likely a random observation.
Microcontroller-based camera traps
At a base cost of 16 USD (Table 1), microcontroller-based camera traps proved to be a cost-effective means to document the presence and foraging behavior of arthropods in the understory of a tropical rainforest. These small and affordable camera traps were able to capture images at regular intervals through the day, facilitating the simultaneous automated photographic documentation of foragers to multiple resin sources over an extended period of time, mitigating the often laborious and limited extent of in-situ personal observations. However, certain challenges remain for the successful adoption of microcontroller-based camera traps.
The inability to install an external real-time clock (RTC) on the ESP32-CAM meant that the diurnal period for photo capture was determined with a photo-resistor that took into account ambient light, instead of dawn and dusk timings. Failure of photo-resistors led to camera traps capturing photos even in darkness, which resulted in a reduced runtime average of 60 h. A single battery was sufficient to run the camera trap for an average of 72 h; increasing the number of batteries can thus lead to longer runtimes. Image quality with the 2MP OV2640 camera was decent in good lighting, but deteriorated with increased graininess in the often-poor lighting in the forest understory. A higher resolution camera may be installed on the ESP32 platform, though with the compromise of increased power draw. From the camera trap photos, identities of organisms could be narrowed down where clear morphological traits could be discerned, though it remained too crude for species-level identification of stingless bee species complexes.
In tandem with the utilization of a microcontroller-based camera trap for this study was the adoption of additive fused deposition modeling (FDM) 3D-printing, which allowed for the prototyping and customization of a camera trap housing and tree trunk mounting solution suited for the requirements of this study (Suppl. File S1). We consider the ability of 3D-printing to, e.g., tailor housing and mounting solutions to suit a generic camera trap platform for specific field conditions and study requirements highly important for “next-generation natural history” (Tosa et al. 2021). Moreover, our pilot study highlights microcontroller-based camera traps as a promising toolkit for natural history observations of arthropods as evidenced above.
Concluding remarks
The use of camera traps at resin sources can greatly facilitate non-intrusive remote observations. Neither the predation interactions between assassin bugs and stingless bees, nor the behavior of assassin bug nymphs and adults at resin sources, would have been observable in person (Chui SX, pers. obs.). There is still potential for improving the camera trap design, e.g. to incorporate an additional battery to double the run time to eight days, as well as sourcing for a more reliable photo-resistor. Recently developed microcontrollers such as the ESP32-CAM are an affordable entry into the world of remote camera trapping for arthropods. Such techniques can nowadays be easily co-opted for use in and thus significantly push forward next-generation natural history observations of these skittish or threatened creatures.
Camera trapping at resin sources revealed that stingless bee resin foraging was uncommon at resin wounds in the understory of a primary rainforest in Borneo, though these results may not reflect resin source availability and resin forager visitation patterns at the canopy or emergent layers of the forest. Canopy camera trapping may actually be used to reveal stratum preferences for resin source visitation by stingless bee species, as was previously found for stingless bee pollen resource partitioning (Nagamitsu et al. 1999).
While stingless bee species that foraged from resin sources did so for the day with repeated and regular foraging bouts, resin sources were often abandoned after one day. Only a few stingless bee species used the same resin source for three, and even six consecutive days. These findings seemingly contradict previous findings which observed higher foraging frequencies at natural and artificially induced resin wounds conducted in the Neotropics (Howard 1985) and in a mix of intact primary forest and mixed disturbance forest sites (Leonhardt and Blüthgen 2009). Notably, the rather disturbed secondary forest habitat harbored a comparatively high density of stingless bee colonies (Leonhardt SD, pers. obs.). Frequenting of, aggressive behavior, and also attacks by predators at resin wounds may therefore be directly correlated with the population density of stingless bees in the surrounding habitat. Future research should therefore investigate how the variety and number of resin sources available in the foraging range of stingless bees, as well as their population density, relate to their resin foraging patterns. For example, additional camera trapping or further observations of stingless bee resin foraging choices could be conducted in less intact habitats, such as secondary forests and forest patches. Knowledge of how resin source availability is influenced by location and habitat degradation is important toward a comprehensive understanding of how anthropogenic changes affect the availability of resources necessary for stingless bees and, consequently, their long-term survival.
Data availability
Data are available on request from the authors.
References
Ashton PS (1964) Manual of the Dipterocarp Trees of Brunei State. Oxford University Press, Oxford
Ashton PS (1968) A Manual of the Dipterocarp Trees of Brunei State and Sarawak: supplement, vol 1. Sarawak Forest Department
Barbosa FM, de Oliveira Campos LA, da Paixão JF, de Oliveira Alves RM (2016) Foraging pattern and harvesting of resources of subterranean stingless bee Geotrigona subterranea (Friese, 1901) (Hymenoptera: Apidae: Meliponini). Papéis Avulsos Zool São Paulo 56:151–157. https://doi.org/10.11606/0031-1049.2016.56.12
Biesmeijer JC (1997) The organisation of foraging in stingless bees of the genus Melipona: An individual-oriented approach. Universiteit Utrecht
Chui SX, Keller A, Leonhardt SD (2021) Functional resin use in solitary bees. Ecol Entomol. https://doi.org/10.1111/een.13103
Danaher MW, Ward C, Zettler LW, Covell CV Jr (2019) Pollinia removal and suspected pollination of the endangered Ghost Orchid, Dendrophylax lindenii (Orchidaceae) by various Hawk Moths (Lepidoptera: Sphingidae): Another mystery dispelled. Fla Entomol 102:671–683. https://doi.org/10.1653/024.102.0401
de Bruijn LLM, Sommeijer MJ (1997) Colony foraging in different species of stingless bees (Apidae, Meliponinae) and the regulation of individual nectar foraging. Insectes Soc 44:35–47. https://doi.org/10.1007/s000400050028
Droissart V, Azandi L, Onguene ER et al (2021) PICT: A low-cost, modular, open-source camera trap system to study plant–insect interactions. Methods Ecol Evol 12:1389–1396. https://doi.org/10.1111/2041-210X.13618
Dykes AP (2000) Climatic patterns in a tropical rainforest in Brunei. Geogr J 166:63–80. https://doi.org/10.1111/j.1475-4959.2000.tb00007.x
Fennell M, Beirne C, Burton AC (2022) Use of object detection in camera trap image identification: Assessing a method to rapidly and accurately classify human and animal detections for research and application in recreation ecology. Glob Ecol Conserv 35:e02104. https://doi.org/10.1016/j.gecco.2022.e02104
Grüter C (2021) Stingless Bees: their behaviour, ecology and evolution. Springer, Cham
Howard JJ (1985) Observations on resin collecting by six interacting species of stingless bees (Apidae Meliponinae). J Kans Entomol Soc 58:337–345
Inoue T, Salmah S, Abbas I, Yusuf E (1985) Foraging behavior of individual workers and foraging dynamics of colonies of three Sumatran stingless bees. Res Popul Ecol 27:373–392
Janzen DH (1985) Insects. In: Janzen DH (ed) Costa Rican natural history, vol 3. impr. Univ. of Chicago Pr, Chicago, pp 619–779
Johnson LK (1983) Foraging strategies and the structure of stingless bee communities in Costa Rica. In: Jaisson P (ed) Social Insects in the Tropics 2. Université Paris-Nord, Paris, pp 31–58
Johnson LK, Hubbell SP (1974) Aggression and competition among stingless bees: field studies. Ecology 55:120–127. https://doi.org/10.2307/1934624
Jolles JW (2021) Broad-scale applications of the Raspberry Pi: a review and guide for biologists. Methods Ecol Evol 12:1562–1579. https://doi.org/10.1111/2041-210X.13652
Kampstra P (2008) Beanplot: A boxplot alternative for visual comparison of distributions. J Stat Softw. https://doi.org/10.18637/jss.v028.c01
Langenheim JH (2003) Plant Resins: Chemistry, Evolution, Ecology, Ethnobotany. Timber Press, Portland
Leonhardt SD, Blüthgen N (2009) A sticky affair: Resin collection by Bornean stingless bees. Biotropica 41:730–736. https://doi.org/10.1111/j.1744-7429.2009.00535.x
Leonhardt SD, Dworschak K, Eltz T, Blüthgen N (2007) Foraging loads of stingless bees and utilisation of stored nectar for pollen harvesting. Apidologie 38:125–135. https://doi.org/10.1051/apido:2006059
Leonhardt SD, Zeilhofer S, Blüthgen N, Schmitt T (2010) Stingless bees use terpenes as olfactory cues to find resin sources. Chem Senses 35:603–611. https://doi.org/10.1093/chemse/bjq058
Meijer W, Wood GHS (1964) Dipterocarps of Sabah (North Borneo). Sabah Forest Department, Sandakan
Michener CD (1961) Observations on the nests and behavior of Trigona in Australia and New Guinea (Hymenoptera, Apidae). Am Mus Novit no. 2026
Mohammed F, Starr CK (1999) Comparative foraging of the sympatric stingless bees Trigona niga and Partamona nigrior (Apidae: Meliponini). In: Proceedings of the section Experimental and Applied Entomology of the Netherlands Entomological Society (N.E.V.) 10: 195–202
Nagamitsu T, Momose K, Inoue T (1999) Preference in flower visits and partitioning in pollen diets of stingless bees in an Asian tropical rain forest. Res Popul Ecol 41:195–202. https://doi.org/10.1007/s101440050023
Norouzzadeh MS, Nguyen A, Kosmala M et al (2018) Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1719367115
Ríos-Saldaña CA, Delibes-Mateos M, Ferreira CC (2018) Are fieldwork studies being relegated to second place in conservation science? Glob Ecol Conserv 14:e00389. https://doi.org/10.1016/j.gecco.2018.e00389
Roepke W (1932) Über “Harzwanzen” von Sumatra und Java. Misc Zool Sumatrana 68:1–5
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge
Roubik DW (2006) Stingless bee nesting biology. Apidologie 37:124–143. https://doi.org/10.1051/apido:2006026
Rovero F, Zimmermann F, Bersi D, Meek P (2013) “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Ital J Mammal 24:148–156. https://doi.org/10.4404/hystrix-24.2-8789
RStudio Team (2016) Rstudio: integrated development for R. Accessed on 6 August 2022
Shanahan M, Spivak M (2021) Resin use by stingless bees: a review. Insects. https://doi.org/10.3390/insects12080719
Symington CF (2004) Forester’s manual of dipterocarps, 2nd edn. Forest Research Institute Malaysia, Kuala Lumpur
Tosa MI, Dziedzic EH, Appel CL et al (2021) The rapid rise of next-generation natural history. Front Ecol Evol 9:698131. https://doi.org/10.3389/fevo.2021.698131
Usinger RI (1958) Harzwanzen or “resin bugs” in Thailand. Pan-Pac Entomol 34:52
Acknowledgements
We thank the Office of Assistant Vice Chancellor (Research) of Universiti Brunei Darussalam (UBD) for the approval of this project, and site access to Kuala Belalong Field Studies Centre; Dr. Kamariah Abu Salim (UBD), Dr. Faizah binti Haji Metali (UBD), Dr. Sylvester Tan (Smithsonian Institute), and Dr. Stuart J. Davies (Smithsonian Institute) as principal scientists of the Kuala Belalong 25 ha Forest Dynamics Plot, for provisioning tree measurement and positional data of dipterocarp trees within the plot; Ms Hajah Masnah binti Haji Mirasan (UBD) for facilitating field station logistics; and Ms Alya Sabrina binti Muhd. Hanbali ‘Sawai’, for her field knowledge as our field assistant at Kuala Belalong. Finally, we are also grateful for the constructive feedback provided by two anonymous reviewers.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chui, S.X., Wahab, R.B.H.A. & Leonhardt, S.D. Stingless bee (Apidae: Meliponini) foraging and predation at trunk resin sources: Rare observations captured with microcontroller-based camera traps in a lowland dipterocarp forest. Insect. Soc. 70, 29–41 (2023). https://doi.org/10.1007/s00040-022-00889-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-022-00889-x