Abstract
Objectives
COVID-19 has a varied clinical presentation. Elderly patients with comorbidities are more vulnerable to severe disease. This study identifies specific symptoms and comorbidities predicting severe COVID-19 and intensive care unit (ICU) admission.
Methods
A literature search identified studies indexed in MEDLINE, EMBASE and Global Health before 5th March 2020. Two reviewers independently screened the literature and extracted data. Quality appraisal was performed using STROBE criteria. Random effects meta-analysis identified symptoms and comorbidities associated with severe COVID-19 or ICU admission.
Results
Seven studies (including 1813 COVID-19 patients) were included. ICU patients were older (62.4 years) than non-ICU (46 years), with a greater proportion of males. Dyspnoea was the only symptom predictive for severe disease (pOR 3.70, 95% CI 1.83–7.46) and ICU admission (pOR 6.55, 95% CI 4.28–10.0). COPD was the strongest predictive comorbidity for severe disease (pOR 6.42, 95% CI 2.44–16.9) and ICU admission (pOR 17.8, 95% CI 6.56–48.2), followed by cardiovascular disease and hypertension.
Conclusions
Dyspnoea was the only symptom predictive for severe COVID-19 and ICU admission. Patients with COPD, cardiovascular disease and hypertension were at higher risk of severe illness and ICU admission.
Similar content being viewed by others
Introduction
The ongoing novel coronavirus (COVID-19) pandemic, involving a virus originally identified in Wuhan, China, has forced several countries to take unprecedented public health measures. As health professionals and policymakers try to shield those at highest risk, better defining the risk factors for severe COVID-19 has been identified as an urgent research priority (Cowling and Leung 2020; Lipsitch et al. 2020).
COVID-19 has a seemingly variable clinical presentation and progression. According to data from China, an estimated 10–15% of mild cases progress to severe, and 15–20% of severe cases go on to become critical, with many of those in the latter category requiring treatment in intensive care units (ICU) (Wu and McGoogan 2020). To maximise the use of scarce health system resources, it is imperative to identify those at risk of progressing to severe and critical illness with COVID-19. It is thought that age and underlying chronic conditions (or comorbidities) increase the risk of severe COVID-19 (Centers for Disease Control and Prevention 2020a), but the differential effect of various comorbidities remains unclear, and current guidance is largely based on expert consensus rather than observational data.
In order to build on existing knowledge, a better understanding of the symptoms and comorbidities (which are the first and most routinely collected components of patient data) related to COVID-19 severity is required. There have been a number of meta-analyses on COVID-19 severity conducted to date (Henry and Lippi 2020; Yang et al. 2020a; Li et al. 2020a), but none of these investigated the predictive value of symptoms, nor separated severe disease from ICU admission (which represents the upper end of severity) when investigating the impact of comorbidities. Through separately analysing patients admitted to ICU, this study aims to better assess the most important symptoms and comorbidities for those with very severe (or critical) disease. This will help to inform public health actions aiming to protect the most vulnerable from acquiring COVID-19 and may also guide efforts on early intervention and resource allocation, through informing the design of clinical pathways and risk stratification tools. Deaths from COVID-19 can also be investigated as an outcome of very severe (or critical) disease. But, if done through routine sources (for instance, using death certificates), this would not allow an analysis of predictive symptoms. Moreover, since many patients admitted to ICU with COVID-19 survive, predictors for death may differ from those for ICU admission.
The primary aim of this study was to conduct a systematic review and meta-analysis, aggregating all available data from published studies, of symptoms and comorbidities predictive for severe disease and ICU admission with COVID-19.
Methods
Retrieval of studies
This study was prospectively registered on PROSPERO (CRD42020172126). Identification of relevant existing literature was performed by an online search in three databases: MEDLINE, EMBASE and Global Health, for studies published from 1st January 2019 to 5th March 2020. The MESH headings (keywords) searched were ‘nCoV*’ or ‘coronavirus’ or ‘SARS-2-CoV’ or ‘COVID*’ and ‘symptom*’ or ‘clinical’ or ‘predict*’ or ‘characteristic*’ or ‘co-morbidit*’ or ‘comorbidit*’ or ‘condition*’. Two reviewers (VG, JMY) independently screened the list of titles and abstracts, and the full text of chosen manuscripts. Disagreements on which manuscripts to include during both title and abstract screen, and the subsequent full-text analysis, were discussed until a conclusion was reached. In addition to the MEDLINE/EMBASE/Global Health search, citation tracking was used to identify any remaining relevant published studies, though none were identified. Unpublished and preprint studies were not retrieved due to uncertain data quality.
Inclusion and exclusion criteria
All studies evaluating individual symptoms and comorbidities in predicting severe infection (as measured by disease severity criteria, or ICU admission) were included. All studies of any design, from any time since the outbreak started (in December 2019), were eligible, except case reports of individual patients or literature reviews. To avoid selection bias, no subjective quality criteria were applied to the studies for inclusion. Exclusion criteria included: (1) studies of exclusively paediatric or pregnant patients, due to the varying presentation of COVID-19 in these groups, (2) insufficient data on symptoms/comorbidities on admission in either severe or non-severe disease groups (or ICU and non-ICU groups), (3) coronavirus strains other than COVID-19 and (4) studies not written in English, because of practical limitations with translation.
Data extraction
Two reviewers independently extracted data from the included studies for both narrative synthesis and statistical analysis. From each study, various details including the study population, investigated predictive symptoms or comorbidities, and the definitions used to measure outcomes, were extracted into Microsoft Excel. These details are presented by study in Table 1. The number of patients in each study, both with and without each symptom or comorbidity, was extracted for statistical analysis (described below).
Predictors and outcomes
The symptoms or comorbidities presented were investigated in at least three included studies. Where studies measured symptoms ambiguously (including abdominal pain/diarrhoea (Li et al. 2020b), myalgia/fatigue (Huang et al. 2020) and nausea/vomiting (Guan et al. 2020)), these data were excluded. Some studies reported heart disease and stroke separately (Guan et al. 2020; Wang et al. 2020; Zhang et al. 2020). To allow comparability between studies for meta-analysis, these were grouped into a single predictor (cardiovascular disease). One study was excluded from the analysis of dyspnoea as a predictor of severity, as dyspnoea was part of the definition for severity used by the authors (Tian et al. 2020). Smoking was not included in meta-analyses as the three papers including data on smoking (Guan et al. 2020; Huang et al. 2020; Zhang et al. 2020) were distributed across both severe and ICU groups, rather than reporting consistently on one (or both) of these outcome measures.
For disease severity, the included studies varied in their differentiation of patients’ disease status, with classifications of ‘mild, moderate, severe and critical’ (Xu et al. 2020), ‘ordinary and severe/critical’ (Li et al. 2020b), ‘common and severe’ (Tian et al. 2020) and ‘non-severe and severe’, disease (Guan et al. 2020; Zhang et al. 2020). The first outcome measure used was severe (including both severe and critical cases) versus non-severe disease. For ICU admission, the included studies varied in their definition of ICU admission, with classifications of ‘ICU, mechanical ventilation or death and non-ICU’ (Guan et al. 2020), and ‘ICU and non-ICU’ (Huang et al. 2020; Wang et al. 2020). The second outcome measure used was ICU admission (including ICU, mechanical ventilation or death, where data were grouped together) versus non-ICU admission.
Statistical analysis
Patient numbers were aggregated across all included studies for each group included in the meta-analysis. Gender was compared between groups using the Chi-square test in STATA (StataCorp. 2015). This was not possible for age due to a lack of individual-level data. The predictive value of symptoms and comorbidities for each of severe disease and ICU admission was estimated with random effects meta-analysis in STATA. Random effects models were used to account for between study heterogeneity (Hedges and Vevea 1998), which was estimated with Tau-squared. This provided a pooled odds ratio (pOR), 95% confidence intervals and a p value, for each symptom or comorbidity. A p value of < 0.05 was used as a marker for evidence of significant association.
Preprint
An early version of this manuscript was uploaded to the ‘medRxiv’ preprint server in order to contribute to the rapidly evolving scientific knowledge on COVID-19 (Jain and Yuan 2020).
Results
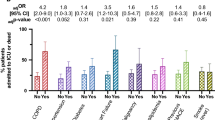
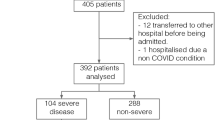
The PRISMA flow diagram (Fig. 1) illustrates the process for selection of papers in this study.
Literature search
The initial search on MEDLINE, EMBASE and Global Health produced 2259 results. After removing duplicates and applying exclusion criteria, there were 42 papers meeting our criteria from title and abstract analysis. On further review, the majority of these studies did not compare proportions of patients with symptoms or comorbidities between severe (or ICU admitted) and non-severe disease (or non-ICU admitted) groups. The reasons for all study exclusions are outlined in Fig. 1. A total of seven studies were selected for inclusion.
Description of included studies
Table 1 shows details of all included studies including reported findings pertaining to symptoms and comorbidities related to severe disease or ICU admission. These seven studies reported on a total of 1813 patients. All included studies were retrospective cohort studies in design, conducted between December 2019 and February 2020 in China, during the novel coronavirus (SARS-CoV-2) outbreak. Guan et al. (2020) conducted the largest study with 1099 COVID-19 patients, whilst Huang et al. (2020) included 40 patients in their study. The number of symptoms investigated varied from five in one study (Tian et al. 2020) to 14 in others (Guan et al. 2020; Wang et al. 2020). The range of comorbidities investigated varied greatly with two studies not including any (Tian et al. 2020; Xu et al. 2020) and one including 22 comorbidities (Zhang et al. 2020).
Table 2 displays the median age and gender of severe and non-severe disease, and ICU and non-ICU admitted, patients, after aggregating all studies. The median age was 62.4 years for ICU-admitted patients compared to 46 years for non-ICU patients, and 49.4 years for severe compared to 41.7 years for non-severe disease patients.
Quality of included studies
All included studies were retrospective cohort studies and were critically appraised using the STROBE checklist (von Elm et al. 2007). The 22 items on the STROBE checklist were formulated into 47 individual indicators, against which each study was marked. The proportion of included studies which met each individual appraisal indicator is illustrated in the online resource (Figure S1). Each paper was assigned an overall quality score based on the percentage of STROBE checklist criteria met (< 55% = −, 55–65% = +, > 65% = ++), as outlined in Table 1.
Appraising with the STROBE checklist highlighted several major weaknesses in the included studies. Firstly, there was no consistent definition on what constituted severe disease. The WHO-China Joint Mission on COVID-19 defined a severe case as tachypnoea (≥ 30 breaths/min) or oxygen saturation ≤ 93% at rest, or PaO2/FiO2 < 300 mmHg (WHO-China Joint Mission 2020). Critical cases were defined as respiratory failure requiring mechanical ventilation, shock or other organ failure that requires intensive care. Although the above criteria were used in some included studies (Xu et al. 2020; Li et al. 2020a), many defined only one out of severe and critical, with one (Li et al. 2020b) using a definition for a single severe/critical cohort. One study reported both severe and critical cases (Guan et al. 2020), using criteria set by the American Thoracic Society. Secondly, the time at which severity of disease was determined was not always clear. Severity was assessed on admission in two studies (Guan et al. 2020; Zhang et al. 2020), whilst three studies did not specify when severity was assessed (Li et al. 2020b; Tian et al. 2020; Xu et al. 2020). It is possible, therefore, that the non-severe group included patients who went on to later develop severe disease. Thirdly, the time point at which symptoms were measured varied from illness onset (via recall) (Huang et al. 2020; Wang et al. 2020; Zhang et al. 2020; Tian et al. 2020) to clinical presentation (Guan et al. 2020; Xu et al. 2020). In one study, it was not clear when symptoms were measured (Li et al. 2020b). Finally, no study specified how each individual symptom or comorbidity was measured. For instance, it was unclear whether fever was objectively measured, and if so, how or by whom.
Meta-analysis
Table 3 shows the odds ratios, 95% confidence intervals and p values for the individual symptoms and comorbidities that were investigated in at least three of the included studies, for both severe COVID-19 and ICU admission, respectively. Forest plots of the predictive symptoms and comorbidities for both severe COVID-19 and ICU admission are illustrated in Fig. 2. A total of seven symptoms were included in the model for severe disease and six for ICU admission, as well as four comorbidities in both. The most prevalent symptoms in the severe group were cough (70.5%), fever (64.1%) and fatigue (44.5%); in the ICU group these were cough (67.2%), fever (62.9%) and dyspnoea (61.2%). The most prevalent comorbidities in the severe group were hypertension (25.4%) and diabetes (16.8%) and in the ICU group were hypertension (40.5%) and cardiovascular disease (CVD) (24.1%).
Men were 1.55 times more likely than women to be admitted to ICU (95% CI 1.02–2.36) but not at a significantly increased risk of being in the severe group. Dyspnoea was the only symptom significantly associated with both severe disease (pOR 3.70, 95% CI 1.83–7.46) and ICU admission (pOR 6.55, 95% CI 4.28–10.0), being more strongly associated with the latter. Cough was associated with severe disease (pOR 1.63, 95% CI 1.03–2.60), but not ICU admission. The remaining symptoms analysed were not associated with either outcome. Chronic obstructive pulmonary disease (COPD), CVD and hypertension were the comorbidities significantly predictive for both severe disease and ICU admission. The pORs for severe disease were as follows: COPD (6.42, 95% CI 2.44–16.9), CVD (2.70, 95% CI 1.52–4.80) and hypertension (1.97, 95% CI 1.40–2.77). COPD, CVD and hypertension were more strongly associated with ICU admission, compared with severe disease, with pORs of 17.8 (95% CI 6.56–48.2), 4.44 (95% CI 2.64–7.47), and 3.65 (95% CI 2.22–5.99), respectively. In contrast, diabetes was of borderline significance in predicting severe disease (pOR 3.12, 95% CI 1.0–9.75), but not ICU admission (pOR 2.72, 95% CI 0.70–10.6), although the Tau-squared value for the latter was unusually high implying a high level of heterogeneity.
Discussion
Key findings
COVID-19 severity was not consistently defined across included studies. All studies were of adequate quality, considering the context, and two were of relatively high quality. The ICU group were older compared to the non-ICU group, with a significantly higher proportion of males.
The most prevalent symptoms in the severe disease group were cough, fever and fatigue and in the ICU admitted group were cough, fever and dyspnoea. The most prevalent conditions in the severe group were hypertension and diabetes and in the ICU group were hypertension and CVD. Males were not at an increased risk of severe disease, but 1.55 times more likely to have an ICU admission compared to females. Dyspnoea was the only symptom significantly associated with disease severity and ICU admission, alongside various comorbidities (COPD, CVD and hypertension). All of these factors were more strongly associated with ICU admission than disease severity. Patients with dyspnoea were 6.6 times more likely to have an ICU admission compared to those without dyspnoea. Although COPD was relatively uncommon, even in ICU patients, it was by far the most strongly predictive comorbidity for ICU admission. Those with CVD and hypertension were 4.4 and 3.7 times more likely to have an ICU admission, respectively, compared to patients without the comorbidity.
Building on existing knowledge
Consistent with Sun et al.’s (2020) meta-analysis of symptoms in 50,466 COVID-19 patients (Sun et al. 2020) and the WHO-China joint mission on COVID-19 (WHO-China Joint Mission 2020), cough and fever were the most common symptoms found in our analysis. We found that the prevalence of dyspnoea in the ICU group was 67.2%, compared with 10.2% in the non-ICU group. Although dyspnoea by definition may be indicative of lung involvement and therefore more severe disease, there have been reports of ‘silent hypoxia’, where oxygen saturations can fall and precipitate acute respiratory failure in the absence of dyspnoea and other symptoms of respiratory distress (Gattinoni et al. 2020). The significant association of dyspnoea in this analysis with severe disease and ICU admission suggests that most patients who progress to severe illness do not have ‘silent hypoxia’. It should be noted though, that the reported strength of dyspnoea as a predictor for severe disease and ICU admission could be affected by selection bias since patients without dyspnoea (but with severe disease) may be less likely to be admitted to hospital in the first instance. The median time from illness onset to dyspnoea is 5–8 days, and the median time from illness onset to ICU admission has been reported as 10–12 days (Centers for Disease Control and Prevention 2020b). One of the studies included in our meta-analysis found patients admitted to ICU had a longer duration of symptoms before hospitalisation compared to those not requiring ICU care (Wang et al. 2020). Given the importance of dyspnoea in predicting ICU admission, future research should aim to assess the value of early hospital admission, clinical intervention and close community-based monitoring in patients with dyspnoea.
The findings reported here are in keeping with current knowledge that the elderly and those with comorbidities are more susceptible to severe infection (Centers for Disease Control and Prevention 2020b; WHO-China Joint Mission 2020). A previous systematic review of 19 prevalence studies, including 2874 patients, found hypertension (18.6%) and cardiovascular disease (14.4%) were the most common comorbidities in COVID-19 patients (Rodriguez-Morales et al. 2020). Our study found similar results with high rates of these comorbidities in severe and ICU groups. For diabetes, a meta-analysis of six studies (1527 patients) found the prevalence to be twice as high in the ICU/severe group compared to non-severe COVID-19 patients (Li et al. 2020a). On disaggregating this outcome into two (severe disease and ICU admitted), we found the prevalence of diabetes to be 2.9 times higher in the severe group (compared to non-severe) and 3.6 times higher in the ICU group (compared to non-ICU).
Yang et al. (2020a) performed a meta-analysis of seven studies including 1576 COVID-19 patients. When comparing severe against non-severe patients, the pooled odds ratios of hypertension, respiratory system disease and cardiovascular disease were 2.36 (95% CI 1.46–3.83), 2.46 (95% CI 1.76–3.44) and 3.42 (95% CI 1.88–6.22), respectively. Although we similarly found that comorbidities were not uniform in terms of the risk of severe COVID-19, COPD was an extremely strong predictor for both severe disease and ICU admission—this latter outcome was not investigated separately in the Yang et al. paper (Yang et al. 2020a).
We demonstrate that various factors are more strongly associated with ICU admission (representing the very severe end of the disease severity spectrum) compared with less severe disease. The major exception to this was gender. Being male was predictive for ICU admission but not severe disease, with similar proportions of males in both severe and non-severe groups. Given that 70% of 2249 intensive care patients in the UK are male (Intensive care national audit & research centre 2020), and that, of the patients who have died from COVID-19 in Italy, 80% were male (Remuzzi and Remuzzi 2020), this is consistent with empirical data. This suggests that hospitalised COVID-19 patients who are male and have severe disease may be at an increased risk of clinical deterioration requiring ICU admission.
Limitations
The foremost limitation of this study was an inability to carry out a multi-variable analysis to account for the presence of several symptoms, comorbidities and potential confounders, such as age. Although this outbreak has seen the evolution of linked data and large open access datasets (Wu and McGoogan 2020) which would be suitable for multi-variable analysis, these currently lack the quality of published data: there are large amounts of missing data, a narrow range of collected variables, and uncertainty about data collection methods and consistency. Our univariable analysis is therefore valuable in evaluating specific individual symptoms and comorbidities predictive for COVID-19 severity and ICU admission using high-quality evidence in the form of peer-reviewed studies.
Secondly, the studies included here were all from China, so the generalisability of findings to other countries and populations is not clear. The Chinese may differ to other populations in terms of their health-seeking behaviour, symptom reporting, prevalence of different comorbidities, as well as their access to high-quality health services. Moreover, because the criteria for ICU admission depends on multiple factors, including bed capacity, this may also differ in other countries, health systems, and at different phases of the epidemic. Nonetheless, given the current dearth of contextually specific evidence available, our findings will help to inform future research and actions.
Finally, it was not possible to account for the timing of presentation in the statistical analysis. If a patient presented after many days of being symptomatic, this may have affected disease severity, compared with an earlier clinical presentation. However, this limitation does not apply to comorbidities, and Table 1 shows information from individual studies on median duration of symptoms before admission, which appears similar between severe (or ICU) and non-severe (or non-ICU) cases. It is therefore unlikely that this will have significantly biased the overall results.
Implications for clinical practice/public health
By identifying the symptoms and comorbidities predictive for severe disease and ICU admission, clinicians can better stratify the risk of individual patients, as early as their initial contact with health services. This can lead to practical changes in management, which can improve allocative efficiency as well as clinical outcomes, through the consideration of more intensive environments of care (e.g. high dependency unit), earlier on, for patients at highest risk of severe infection. These can also be formalised within risk stratification tools to aid clinical decision-making, such as the CURB65 tool for community-acquired pneumonia (Lim et al. 2003). As the number of hospitalised COVID-19 cases continues to increase, hospitals will increasingly need to ration limited resources and improve clinical pathways to effectively prioritise patients with greatest clinical need. Anticipation of future demand, based on local population characteristics, may enable more timely planning and resource mobilisation (Yang et al. 2020b). Identifying those at the highest risk will also facilitate better-informed discussions between clinicians, patients and patients’ families about the anticipated clinical trajectory, allowing more accurate and timely advance care planning to occur.
Identifying those at high-risk will aid the public health response in controlling the spread of disease. Given the ubiquity of comorbidities in the elderly population, and their increased susceptibility to severe COVID-19 infection (WHO-China Joint Mission 2020), knowledge on the differing prevalence and risk of various conditions may help to focus and tailor public health efforts. For instance, for COPD, which is less common in the general population and very strongly associated with ICU admission, a more targeted and intensive health protection strategy may be warranted, compared to other conditions (such as hypertension) which are more difficult to target due to their higher prevalence in the general population.
Furthermore, if it is found that severity of illness is related to infectivity, as is the case in the closely related SARS-CoV, then identifying patients who may develop severe illness can help guide precautions to prevent the spread of SARS-CoV-2. These include infection control decisions regarding the limited availability of isolation rooms and personal protective equipment (PPE), particularly in more resource-constrained settings.
Implications for future research
Specific guidance for future observational cohort studies investigating factors predictive for severe COVID-19 is outlined in Table 4. This is a list of measures researchers can take to improve the quality of research and therefore the utility of study findings. These recommendations are indicative rather than exhaustive and have been made based on the number of limitations identified in the design of studies included in this review. This may not apply to studies investigating different predictive factors or outcomes to those reported here and does not account for the practical or resource constraints researchers may face.
Conclusions
Being male was predictive for ICU admission but not severe disease, suggesting that hospitalised COVID-19 patients who are male and have severe disease may be at an increased risk of clinical deterioration. Dyspnoea was the only symptom strongly predictive for both severe disease and ICU admission and could be a useful symptom to help guide risk assessment and timely clinical management. The association between comorbidities and severe disease was not homogenous. In ICU-admitted patients, who represent the more severe end of the spectrum of clinical severity, the difference in effect sizes for COPD and the other included comorbidities was large, suggesting that COPD patients are particularly vulnerable to very severe (or critical) disease. As the outbreak develops, future research must build on these findings by investigating factors related to disease severity, including a wide range of comorbidities and the effect of various potential confounding factors. This will aid clinical assessment, risk stratification and resource allocation and allow public health interventions to be targeted at the most vulnerable.
Availability of data and material
All data are fully available from the corresponding author on reasonable request.
References
Centers for Disease Control and Prevention (2020a) People who are at higher risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html. Accessed 25 April 2020
Centers for Disease Control and Prevention (2020b) Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed 25 April 2020
Cowling BJ, Leung GM (2020) Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Eurosurveillance 25:1–5. https://doi.org/10.2807/1560-7917.ES.2020.25.6.2000110
Gattinoni L, Chiumello D, Caironi P et al (2020) COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. https://doi.org/10.1007/s00134-020-06033-2
Guan W-J, Ni Z-Y, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032
Hedges LV, Vevea JL (1998) Fixed- and random-effects models in meta-analysis. Psychol Methods 3:486–504. https://doi.org/10.1037/1082-989X.3.4.486
Henry BM, Lippi G (2020) Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2019:9–10. https://doi.org/10.1007/s11255-020-02451-9
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Intensive care national audit & research centre (2020) ICNARC report on COVID-19 in critical care. 4 April 2020
Jain V, Yuan J-M (2020) Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. medRxiv. https://doi.org/10.1101/2020.03.15.20035360
Li B, Yang J, Zhao F et al (2020a) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109:531–538. https://doi.org/10.1007/s00392-020-01626-9
Li K, Wu J, Wu F et al (2020b) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000672
Lim WS, van der Eerden MM, Laing R et al (2003) Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58:377–382. https://doi.org/10.1136/thorax.58.5.377
Lipsitch M, Swerdlow DL, Finelli L (2020) Defining the epidemiology of Covid-19—studies needed. N Engl J Med 382:1194–1196. https://doi.org/10.1056/NEJMp2002125
Remuzzi A, Remuzzi G (2020) COVID-19 and Italy: what next? Lancet 395:1225–1228. https://doi.org/10.1016/S0140-6736(20)30627-9
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E et al (2020) Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. https://doi.org/10.1016/j.tmaid.2020.101623
StataCorp. (2015) Stata Statistical Software: Release 14
Sun P, Qie S, Liu Z et al (2020) Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol 92:612–617. https://doi.org/10.1002/jmv.25735
Tian S, Hu N, Lou J et al (2020) Characteristics of COVID-19 infection in Beijing. J Infect 80:401–406. https://doi.org/10.1016/j.jinf.2020.02.018
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Int Med 147:573. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. https://doi.org/10.1001/jama.2020.1585
WHO-China Joint Mission (2020) Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 323:1239. https://doi.org/10.1001/jama.2020.2648
Xu Y-H, Dong J-H, An W et al (2020) Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 80:394–400. https://doi.org/10.1016/j.jinf.2020.02.017
Yang J, Zheng Y, Gou X et al (2020a) Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis 94:91–95. https://doi.org/10.1016/j.ijid.2020.03.017
Yang X, Yu Y, Xu J et al (2020b) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2600:1–7. https://doi.org/10.1016/S2213-2600(20)30079-5
Zhang J, Dong X, Cao Y et al (2020) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 1:14238. https://doi.org/10.1111/all.14238
Funding
The authors received no specific funding for this work
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Both authors performed material preparation, data collection and analysis and both equally contributed to writing the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Human and animal rights
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, V., Yuan, JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health 65, 533–546 (2020). https://doi.org/10.1007/s00038-020-01390-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-020-01390-7