Abstract
Lepidurus arcticus (the Arctic tadpole shrimp) is a vulnerable keystone species in Arctic and alpine water bodies where its occurrence and population size may influence the viability and life history traits of resident salmonids. Using data from a Norwegian mountain hydropower reservoir, Aursjoen, we illustrate how reduced availability of L. arcticus as prey resulted in the reduced condition, growth and delayed maturation of resident brown trout (Salmo trutta). We further link changes in the relative abundance of L. arcticus as prey to changing reservoir conditions, e.g. water level changes in the spring period, thereby establishing an indirect link between reservoir operation regimes and brown trout population traits. While no evidence for decreased brown trout survival was found, the results indicate that alternative brown trout prey resources, i.e. the small chydorid cladoceran Eurycercus lamellatus, do not appear to have successfully offset the caloric loss from reduced consumption of large-sized L. arcticus. Although the fundamental explanation for the observed L. arcticus collapse remains largely unknown, the present findings provide strong evidence that this vulnerable crustacean species can affect the abundance, viability and life history traits of valued resident salmonid populations in oligotrophic alpine lakes and reservoirs exposed to climate- and hydropower-driven changes in water levels and temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improved understanding of relationships between abundance of keystone invertebrate species and life history traits of their predators would facilitate better management and mitigation of biodiversity loss in alpine and high-latitude freshwater ecosystems (Lento et al. 2019). In lakes hosting salmonid fishes, large-sized crustacean species commonly act as keystone species due to their omnivorous diet and high importance for fish nutrition (Sømme 1934; Borgstrøm et al. 1985; MacNeil et al. 1999). Although large-sized crustaceans are known to be a highly nutritious food (Lien 1978; Dararat et al. 2012) and to affect food-web dynamics in lakes (Primicerio and Klemetsen 1999; Jeppesen et al. 2001; Weidman et al. 2011; Eloranta et al. 2013), there is little research on how differences in their relative abundance may affect the life history traits, such as growth and maturation, of apex salmonid predators.
The Arctic tadpole shrimp (Lepidurus arcticus Pallas 1793; Branchiopoda, Nostostraca) is a keystone species commonly occurring in freshwater lakes and ponds throughout the circumpolar sub-Arctic to high-Arctic zones, inhabiting areas from 60° N to 80° N (Rogers 2001; Rautio et al. 2011; Lakka 2013; Coulson et al. 2014). L. arcticus is an indicator species of environmental change (Lakka 2013) and is a red listed species in Finland and Sweden (Westling 2015; Väinölä et al. 2019). L. arcticus is often preyed upon by breeding birds such as Steller's eider (Polysticta stelleri), Arctic tern (Sterna paradisaea), purple sandpiper (Calidris maritima) and dunlin (Calidris alpine) (Quakenbush et al. 2004; Lakka 2013). It is an annual species that grows rapidly from emergence in early summer and is an important prey item for many fish species such as brown trout (Salmo trutta), Arctic charr (Salvelinus alpinus), European minnow (Phoxinus phoxinus), European whitefish (Coregonus lavaretus), broad whitefish (Coregonus nasus), and Arctic grayling (Thymallus arcticus) (Borgstrøm et al. 1985; Haugen and Rygg 1996a; Moulton et al. 2007; Woods et al. 2013, Lakka et al. 2019). In the absence of fish, L. arcticus is commonly among the most abundant benthic invertebrate species in many Arctic ponds and lakes (Christoffersen 2001; Jeppesen et al. 2001), with predation by fishes having been implicated in the marked decline of L. arcticus in many instances (Aass 1969; Jeppesen et al. 2001, Heggen et al. 2010). As a consequence, the absence of L. arcticus has been noted to negatively affect fish populations, with declines in their abundance having been associated with reduced recruitment and growth of fish (Kristensen et al. 2006).
As with many r-selected, short-lived species (Reznick et al. 2002), L. arcticus abundances are variable in time and responsive to predator densities and prevailing environmental conditions. Accordingly, L. arcticus often disappears and reappears as fish densities rise and fall (Aass 1969). Furthermore, they can be extremely sensitive to prevailing environmental conditions, often occurring in very low numbers in late, cold summers as well as in cold-water lakes in Norway (Borgstrøm et al. 2018; Qvenild et al. 2018; Qvenild and Hesthagen 2019). However, as a cold-water stenotherm, L. arcticus might also be affected by warm temperatures (Lakka 2013), with predicted climate-driven increases in water temperatures likely to have detrimental impacts on their abundance and distribution within Norway (Qvenild et al. 2018). For example, the ecophysiological activity of L. arcticus peaks at 10 °C (Lakka 2013; Pasquali et al. 2019) and it has been found in natural waters only as warm as 19 °C (Arnold 1966). Water chemistry, principally pH, also holds implications for the viability of L. arcticus, with slight drops in pH below the critical limit of about 6.0 having the potential to locally extirpate populations (Borgstrøm and Hendrey 1976; Fjellheim et al. 2007; Lakka 2013). Furthermore, in hydropower reservoirs, the amplitude, frequency and timing of water level fluctuations can influence the recruitment and abundance of both L. arcticus and the predatory brown trout likely through decreased food and habitat availability and increased juvenile mortality associated with drying and freezing of shallow littoral areas (Brabrand 2010; Hirsch et al. 2017; Eloranta et al. 2018).
In Norway, and when available, L. arcticus is considered to be one of the most important prey for brown trout populations in alpine lakes and reservoirs (Aass 1969; L’Abée-Lund and Sægrov 1991; Fjellheim et al. 2007). Importance varies both seasonally and by fish size, tending to peak in brown trout diets in August and to be higher in larger fish (Haugen and Rygg 1996a, b). The strength of the prey selection process, particularly among brown trout (Aass 1969), suggests that their occurrence in fish stomachs is a useful surrogate for benthic sampling programs when attempting to determine overall relative L. arcticus abundance within a lake. Indeed, paired fish stomach and benthic sampling studies have shown fish stomach examination to be among the best methods for monitoring low-density populations of prey species such as L. arcticus (Fjellheim et al. 2007; Järvinen et al. 2014; Qvenild et al. 2018; Qvenild and Hesthagen 2019). While useful, the method is sensitive to the timing of sampling, particularly for short-lived species such as L. arcticus that exhibit short generation time. Nevertheless, L. arcticus presence in fish stomachs can be used to study long-term changes in the abundance and occurrence of this keystone species (Einarsson et al. 2004; Fjellheim et al. 2007; Sægrov et al. 2013).
Given the importance of L. arcticus as a prey item for brown trout (Borgstrøm et al. 1985), loss of their availability as prey could hold significant implications for brown trout life history traits, reducing growth and/or condition as a result of reduced energy intake (Lien 1978; Haugen and Rygg 1996a, b; Brabrand 2010). Here, we use data collected from standardized sampling of a Norwegian hydro-electric reservoir to examine the consequences of differences in the relative abundance of L. arcticus, as inferred from stomach contents analysis, for the condition, growth, maturity and survival of resident brown trout. We compare key environmental variables, such as water level fluctuations and air temperature previously linked to L. arcticus population success (Borgstrøm et al. 1985; Lakka 2013; Pasquali et al. 2019). Finally, we compare periods of L. arcticus high (1985–1989) and low (1995–1999) relative abundance and test the hypothesis that low relative abundance and dietary use of L. arcticus is associated with differences in key biological traits such as reduced body condition, growth rate, maturity and survival of reservoir resident brown trout.
Methods
Study area
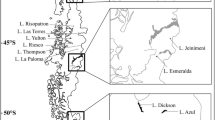
The study was carried out in the Aursjoen reservoir (Fig. 1) which is located in the Reinheimen mountain area at 1097 m a.s.l. in southern-central Norway (61.94° N, 8.27° E) and used as a feed reservoir for hydro-electric power generation (115 × 103 MWh). Given the seasonal nature of hydro-electric generation, the reservoir undergoes an up to 12.5 m annual fluctuation in water level surface elevation, typically being lowest in late spring and highest in late autumn (Online Resource 1). Consequently, the reservoir surface area can vary from 7.4 km2 at high water levels to as little as 2.7 km2 at low water levels, thereby exposing 64% of the reservoir bottom areas to desiccation (Hesthagen 2018). The highest daily water level changes typically occur during or after ice-off in May to June, coinciding with the critical hatching and juvenile development period of L. arcticus (Brabrand 2010; Borgstrøm 2019). The reservoir has a maximum depth of 28 m, mean depth of 7.4 m, is oligotrophic (14 µg L−1 total phosphorus, 48 µg L−1 total nitrogen, 2–5 mg L−1 total organic carbon) with pH in the range 6.2–6.5, and experiences surface temperatures that rarely exceed 15° (Hesthagen 2018, www.vann-nett.no).
Map of Aursjoen reservoir depicting location in relation to southern Norway, aerial photographs from 21 September 2015 and 29 September 2010 depicting the extant of exposed shoreline associated with 2 m water level drawdown (1097.7 m versus 1095.7 m a.s.l., respectively), and absolute daily water level changes (cm) during the study periods 1985–1989 (solid line) and 1995–1999 (broken line). Maps and photos are obtained from www.norgeskart.no and http://www.norgeibilder.no, respectively. The water level data is obtained from a database managed by the Norwegian Water Resources and Energy Directorate (www.nve.no)
Sampling
Brown trout are the only resident fish species in the reservoir. The population has been supplemented annually by hatchery-reared fish (age 0+), with between 2000 and 7000 specimens stocked annually to offset reservoir impoundment effects (Hesthagen 2018). The monitoring of fish stocks has been carried out yearly since 1981 during the late open-water season (August–September) using gillnets (25 × 1.5 m) with knot-to-knot mesh sizes of 39 mm prior to 1999 and 35 mm in subsequent years. The nets were set overnight and randomly distributed throughout the reservoir. All captured fish (n = 3417) were weighed (mass in g), measured (fork-length in mm), sexed and assessed for maturity using a binary classification (mature, immature) based on the state of gonadal development and the presence of developing ova. Scales were removed for aging purposes. Two study periods were selected for comparison: 1985–1989 with high L. arcticus abundance and 1995–1999 with low L. arcticus abundance in brown trout diet.
Stomachs were dissected from a sub-sample of fish each year (n = 20–159) and preserved for subsequent examination in the laboratory. All prey items were identified to the lowest practical taxonomic level (Fig. 2), counted, and measured to the nearest 0.01 mm whenever possible prior to calculation of dry weight using the body length–mass regressions described in L’Abée-Lund and Sægrov (1991) and Breistein and Nøst (1997). The relative contributions of each prey taxon to the estimated total mass of all stomach contents (i.e., sum of all estimated dry weights) were calculated for each fish. Finally, these prey proportions (% dry weight) for individual fish were used for calculation of population-level proportions of different prey taxa, including L. arcticus and the semi-benthic chydorid cladoceran Eurycercus lamellatus, in each year.
Statistical analyses
A before and after statistical design was employed (Smith 2002) to test for an association between L. arcticus and brown trout condition, growth, maturity and survival characteristics. Periods of high (1985–1989) and low (1995–1999) L. arcticus abundances were identified on the basis of gut content analysis (Fig. 2) and separated by a period equivalent to the average historical capture age (5.1 years) to eliminate possible carry-over effects that might bias analyses (Harrison et al. 2011).
Differences in stocking levels between periods were tested using one-way ANOVA to rule out possible confounding effects (Smith 2002) on period-to-period differences in fish life history traits. To account further for the cumulative effects of stocking over the individual lifetimes of fish, stocking was measured as the 5-year average up to the year of capture. Differences in yield (kg ha−1) and catch per unit effort (CPUE), expressed as the number of fish caught per 100 m2 net area per 12 h, between the two periods were similarly tested using one-way ANOVA.
Early season water levels, water level fluctuations and water temperature are suggested to be important for the hatching and development of L. arcticus (Brabrand 2010; Lakka 2013; Pasquali et al. 2019). Therefore, differences between periods in monthly open water season (May through September) daily water level (m a.s.l), absolute daily water level change (absolute water level change from dayt to dayt+1 regardless of sign) and air temperature were assessed for significant differences in mean using the Kruskal-Wallis (K-W) test after assessment for data normality (Zar 2010). A between period comparison of May through September mean water levels was completed by comparing day of the year (DOY) means calculated as the arithmetic average of the water level for a given DOY across the five years within each period and subtracting the low abundance period mean from the high abundance period mean. The resulting positive and negative values, respectively, thus indicate higher and lower average water levels during the high L. arcticus abundance period. The statistical significance of differences between the periods was tested with a paired t test (Zar 2010). Significant differences in fish condition were determined by testing the homogeneity of the estimated weight-length regression slope for the two periods (high and low L. arcticus abundance) within a general linear modelling (GLM) framework (Zar 2010) and further via one-way ANOVA using Fulton’s condition factor (K). Differences in growth were tested by comparing standard von Bertalanffy growth models (Diana 2004) for each L. arcticus abundance period that were estimated using non-linear regression methods (Bates and Watts 1988), with significance established using an analysis of residual sum of squares (Chen et al. 1992; Haddon 2001).
The probability of maturity was modelled as a function of age with logistic regression following methods described in Agresti (2002), with mean age-at-maturity estimated from the model at the 0.5 probability point and significant differences between L. arcticus abundance periods established using an analysis of residual sum of squares (Chen et al. 1992; Haddon 2001). Survival was estimated based on the numbers of aged fish captured each year, with annual survival probabilities estimated using Chapman-Robson methods (Chapman and Robson 1960; Robson and Chapman 1961) beginning with the first fully vulnerable age-class in the catch. Chapman-Robson methods have been shown to out-perform regression estimators, yielding the lowest errors and biases (Dunn et al. 2002). All statistical analyses were performed using the Statistica Release 8 statistical software (Statsoft, Tulsa, OK).
Results
Gut contents of sampled brown trout showed a shift from a diet dominated by L. arcticus and Bythotrephes sp. to one dominated by E. lamellatus and surface insects (Fig. 2). Significant differences were observed in the mean annual relative contribution of the two key prey items (L. arcticus: ANOVA F1, 8 = 190.88, P < 0.001; E. lamellatus: ANOVA F1, 8 = 17.544, P = 0.003) to brown trout stomach contents during the two study periods, with L. arcticus declining from 66.1 to 1.8% and E. lamellatus increasing from 8.5 to 55% between 1985 and 1989 and 1995–1999, respectively (Fig. 2). There was no significant difference in average fish stocking levels between the two periods characterized by high and low L. arcticus abundance (ANOVA F1, 8 = 0.013, P = 0.991), with mean annual values of 5336 and 5264 stocked specimens, respectively.
Between period differences were evident in monthly mean daily reservoir water levels (m a.s.l.), with all monthly comparisons (May through September) showing significant (P ≤ 0.006 for all K-W tests) but minor (0.27–1.26 m) differences that equate to approximately 2–10% (0.1–0.5 km2) of the mean wetted area of the reservoir. Absolute mean daily water level changes differed significantly between the high and low L. arcticus abundance periods in all months (P ≤ 0.003 for all K-W tests) except July (K-W P = 0.495), with changes ranging from lows of 0.01–0.04 m in the period July through September to highs of 0.16–0.25 m in June (Online Resource 2). Moreover, the pattern of differences between periods was most evident throughout the May to September period, with daily mean water levels in the high L. arcticus abundance period being consistently higher in the early May to June period and consistently lower in the later August to September period. Across the May to September period, mean day of the year water levels differed significantly (t = 2.234, P = 0.0027, df = 149) between periods (Fig. 3). Air temperatures differed significantly between periods (high abundance − low abundance) in most months, with June (+ 1.62 °C), August (− 2.23 °C) and September (− 2.19 °C) evidencing significant differences (P ≤ 0.006 for all K-W tests) and May (− 0.29 °C) and July (− 0.55 °C) showing no significant differences (P ≥ 0.091 for all K-W tests). Across months temperatures varied from lows in the 4 °C range in May to highs in the 10 °C range in July before declining to values in the 4–6 °C range in September.
Average calendar day of the year differences in water level between the high and low L. arcticus abundance periods in Aursjoen. Positive values indicate higher average water levels during the high abundance period. Means calculated as the average water level for a given day of the year for all years within each abundance period
Period significantly influenced the slope and intercept of the brown trout weight-length regression (F1, 3414 = 2550.60, P < 0.001), with length (F1, 3414 = 100428.00, P < 0001) being a significant determinant of weight in each period (Table 1, Online Resource 3). Similarly, there was a significant difference in Fulton’s condition factor (K) for fish captured in the high and low L. arcticus abundance periods (F1, 3415 = 2805.6, P < 0.001), with the mean K value falling from 1.17 to 0.99. Differences in condition were evident at all ages (age-4: F1, 1203 = 448.94, P < 0.001; age-5: F1, 1054 = 315.80, P < 0.001; age-6: F1, 423 = 322.87, P < 0.001), for which there were a sufficient number of fish captured to complete robust comparative statistical testing.
Brown trout length-at-age differed significantly (F3, 3414 = 606.94, P < 0.001) between the study periods, with the estimated von Bertalanffy model for the high L. arcticus abundance period lying wholly above the model estimated for the low abundance period (Table 2; Fig. 4). Consistent with condition and growth, the yield (kg ha−1) of brown trout in the L. arcticus high and low abundance periods differed significantly (ANOVA F1, 8 = 81.39, P < 0.001), declining from an average of 1.90 kg ha− 1 to 0.19 kg ha− 1. Similarly, brown trout CPUE (number of fish caught per 100 m2 per 12 h) also differed significantly between periods (ANOVA F1, 8 = 43.38, P < 0.001), declining from 0.43 in the high abundance period to 0.15 in the low abundance period.
Maturity increased more rapidly with age during the high L. arcticus abundance period than in the low abundance period (Fig. 5), with the respective mean age-at-maturity estimates being 5.1 and 7.0 years in the high and low abundance periods. Probability of maturity models differed significantly (F2, 12 = 9.568, P < 0.001) between the study periods.
Within the two study periods, brown trout survival varied among years, ranging from 0.31 to 0.47 at high abundance and 0.28–0.48 at low L. arcticus abundance period. However, mean survival did not vary between the periods (F1, 8 = 0.089, P = 0.773), averaging 0.38 and 0.39 in the high and low L. arcticus abundance periods, respectively.
Discussion
As hypothesized, reservoir resident brown trout evidenced reduced body condition, growth and maturity in years of low L. arcticus abundance, but did not show any significant change in survival. Overall, the results suggest that the effects of reduced L. arcticus acted primarily on the life history traits of the brown trout by reducing somatic growth and gonadal development. In a bioenergetics context (Elliott 1976), the results point to an overall decline in energy intake by brown trout associated with the decreased relative abundance and availability of L. arcticus as prey, causing reduced condition and growth. Coincident with these changes, brown trout yield also declined by on average 1.7 kg ha−1, falling to 10% of long-term historical catches. While survival did not significantly change between periods, the similarly large reductions noted in CPUE are indicative of declines in abundance between periods (Elliott and Fletcher 2001) that coincided with the noted declines in L. arcticus. Observed reductions in growth in all age groups and increases in mean age-at-maturity (here 2 years) would further be expected to reduce overall reproductive potential given the strength of the fecundity–size relationship in brown trout (Elliott 1994; Jonsson and Jonsson 2011).
Our results suggest a link between L. arcticus and the environment having implications for brown trout. L. arcticus has a 1-year life cycle, but their resting eggs can stay viable for several years and tolerate freezing and desiccation, as observed for other crustaceans (Rogers 2014). The successful recruitment and thus availability of L. arcticus as an important summer-time food source for brown trout is highly dependent on successful laying of eggs during the autumn of the previous year, as well as on the successful hatching of eggs and the rapid development of juvenile stages early in the growing season. Water level and temperature during these critical spawning and hatching periods are particularly important for L. arcticus recruitment and abundance due to their effect on dewatering or flushing of deposited eggs, accessibility of littoral spawning and nursing areas, and the development time of early life stages (Borgstrøm et al. 1985; Brabrand 2010; Pasquali et al. 2019). Therefore, delayed or insufficient filling of a reservoir and/or rapid water level fluctuations in spring and autumn may impair successful hatching and spawning of L. arcticus, potentially reducing food availability for resident fish populations (Borgstrøm et al. 1985; Brabrand 2010). In Aursjoen, the relatively higher water levels in May–June (Fig. 3) during the high abundance period (1985–1989) would have yielded conditions favourable to the successful hatch and juvenile recruitment as compared to the low abundance period (1995–1999) when reservoir water levels were as much as 2.5 m lower. Further, the more limited or incomplete reservoir filling characterizing the low L. arcticus abundance period would have reduced availability of important shallow littoral feeding areas for both L. arcticus and the resident brown trout, reducing the availability of the prey to its predator.
In Aursjoen, the low L. arcticus abundance seems to be associated with lower daily water levels in early summer, i.e. during or soon after the critical hatching period between late May and early July when brown trout also feed actively. Due to their rapid growth rate, L. arcticus are preyed upon a few weeks after hatching by brown trout (Borgstrøm 1970) and they provide one of the richest prey energy sources (4.8–5.5 calories mg−1 dry weight) typically found in brown trout stomachs (Lien 1978). The observed heavy reliance by brown trout on L. arcticus has been noted by other studies of mountain Norwegian reservoirs where brown trout prey upon L. arcticus particularly in the July through September period when the bulk of brown trout growth also occurs (Borgstrøm 1973; Borgstrøm et al. 1985; Jonsson and Jonsson 2011). The effects of the potential loss of this high-quality food resource may not be restricted to the fish populations and aquatic environments, because many avian predators also feed on L. arcticus (Quakenbush et al. 2004; Lakka 2013).
Lepidurus arcticus are also sensitive to water level fluctuations (Borgstrøm 1973; Borgstrøm et al. 1985), with the degree of sensitivity linked to the seasonal timing of the fluctuations. L. arcticus lives and hides from predators among mosses and stones in very shallow, < 0.5 m littoral habitats (Lakka 2013) and is likely to be among the first taxa affected by water level fluctuations. Further, higher and more variable water levels may exert greater mechanical action on reservoir ice, thereby promoting earlier break-up and a longer ice-fee period (Stander 2006; Gebre et al. 2013). Loss of ice-cover has been linked to greater littoral warming (Vincent 2009; Brown and Duguay 2010), particularly when associated with warmer spring air temperatures as occurred in the 1985–1989 June period at Aursjoen. In turn, warmer temperatures have been correlated with higher L. arcticus growth in the High Arctic (Lakka 2013), while lower temperatures and reduced ice-free periods have been correlated with lower abundance in southern Norway (Qvenild et al. 2018). Indeed, temperature, pH and water level fluctuations are the main triggers for controlling L. arcticus presence in brown trout diet in reservoirs. Thus, water level fluctuations promoting earlier ice-break up, when followed by increased air temperatures and reduced water level fluctuations during the critical period of L. arcticus emergence and growth, would seem to favour L. arcticus and promote both greater abundance and better feeding conditions for reservoir resident brown trout.
In contrast, periods of reduced water levels in spring, coupled with reduced air temperatures and coincident increases in water level fluctuations during the critical period of L. arcticus emergence and growth, as happened in the 1995–1999 period in Aursjoen, would appear to have the opposite effect and work to reduce L. arcticus abundance and prey availability for brown trout. Even though L. arcticus eggs tolerate drying (Longhurst 1955), they do not hatch on dry land. Therefore, for successful recruitment, the shallow littoral spawning and hatching areas of L. arcticus should be watered in August to September and the following spring when the water temperature is optimal for hatching (5–10 °C). The combination of intensive water level fluctuation during the hatching time and high temperatures in late summer, as observed during the low abundance period, would seem to limit L. arcticus recruitment and survival and its subsequent availability as an optimal food source for brown trout in Aursjoen reservoir. Extended and more variable water levels through the summer foraging period may also have acted to increase reservoir turbidity (Bonalumi et al. 2012) and affected fish foraging efficiency (Borgstrøm et al. 1992; Stuart-Smith et al. 2004) and use of L. arcticus as prey.
Laboratory studies have demonstrated that fish show a clear preference for large prey items (Ringler 1979; Wetterer 1989), which are normally thought to be the most energetically profitable. The feeding strategy of brown trout is flexible and influenced by the size frequency distribution of potential prey. Thus, brown trout will tend to feed on either small numbers of large prey or large numbers of small prey (Sánchez-Hernández and Cobo 2015). In Aursjoen, the loss of the larger L. arcticus as a key prey item was offset by increased consumption of the smaller E. lamellatus, although the utilization of this species does not appear to have successfully offset the caloric loss from reduced consumption of L. arcticus. Using data on the mean dry weight equivalence of L. arcticus obtained from brown trout stomachs multiplied by the caloric value of L. arcticus as prey (Lien 1978) suggests the average caloric value of L. arcticus in sampled brown trout stomachs declined from 368.5 calories in 1985–1989 to 10.9 calories in 1995–1999. The average loss of 357.6 calories was only partially offset by increased use of E. lamellatus whose mean dry weight equivalence in calorie terms equalled 296.7 over the 1995–1999 period. The resulting 16.5% net calorie loss is sufficient to reduce the achievable scope for growth (Elliott 1994) when cumulated over the entire growing season. The ultimate effect of a reduction in calorie intake can be moderated by differences in water temperatures as a result of the noted interaction between temperature and ration (calories consumed) in the determination of brown trout growth (Elliott 1994), with increases in temperature at a given ration tending to reduce the realized growth rate (Elliott 1976).
Further affecting brown trout condition and growth may have been the ecological characteristics of E. lamellatus as the alternative for L. arcticus. Although E. lamellatus is known as brown trout prey and can account for between 31 and 41% of age-1 + brown trout diet, consumption tends to fall in fish larger than 150 mm (Hesthagen et al. 1992). In lakes at the Hardangervidda mountain area in Norway, E. lamellatus is the main prey for small brown trout and is consumed by larger individuals (> 400 mm) if large-sized crustaceans (Lepidurus arcticus and Gammarus lacustris) are absent or scarce (Qvenild and Hesthagen 2020). However, the highest E. lamellatus abundances occur later in the growing season, e.g. September to October (Koksvik 1995) than is observed for L. arcticus that peak as brown trout prey in August–September (Qvenild et al. 2018). Declines in the use of smaller prey with size relates to the energetic advantage obtained by feeding on larger prey (Wankowski and Thorpe 1979), with salmonids tending to grow larger when feeding on larger, more energetically valuable prey (Mittelbach and Persson 1998). A lack of suitably large prey and overall prey availability are thought to constrain fish growth (Kerr 1971; Dodrill et al. 2016), particularly as time spent foraging tends to increase with fish size (Ware 1972). Thus, the foraging gain-cost ratio of E. lamellatus appears to have limited the brown trout scope for growth in the low L. arcticus abundance period in Aursjoen.
Variations in seasonal availability may also act to limit the overall energetic gains obtained by heavy reliance on E. lamellatus. In Norwegian lakes, E. lamellatus has its highest abundance in autumn, e.g. September–October, with the increase in biomass occurring later than the seasonal increases in the water temperatures (Koksvik 1995) that would drive increasing metabolic energy demands in brown trout (Elliott 1976). The abundance of E. lamellatus appears to be connected to the development of littoral zone vegetation used as preferred habitat (Smirnov 1962). In hydro-electric reservoirs where the littoral zone has been degraded by water level regulation (Eloranta et al. 2017; Hirsch et al. 2017), the strong link between the development of littoral zone vegetation and E. lamellatus abundance may limit further their value as replacement prey, particularly as L. arcticus emerge early in spring, grow quickly (Borgstrøm et al. 1985) and can provide suitable energy sources to brown trout early in the growing season.
Lepidurus arcticus is known to be susceptible to environmental changes, having been adversely affected throughout much of its natural range, e.g. by acid rain in southern Norway (Fjellheim et al. 2001). The species is also temperature sensitive (Lakka 2013). Indeed, the timing of ice break-up and the rate of spring warming and water levels have been shown to be significant determinants of the growth and development of L. arcticus (Brabrand 2010; Borgstrøm 2019). Current climate change predictions forecast significant increases in both air temperature and precipitation in Norway (IPCC 2014), with a potential consequence being the localized extinction of cold-adapted crustacean species (Lindholm et al. 2012), including L. arcticus (Lakka 2013; Qvenild et al. 2018; Qvenild and Hesthagen 2019). Localized extinctions will have significant consequences for resident fish species, such as brown trout. Based on the documented experiences of the Aursjoen reservoir, reduction of L. arcticus in other lakes and regulated reservoirs is likely to be associated with lower brown trout growth rates, reduced condition and delayed maturation, which will reduce the fish production and recreational value of the affected water bodies.
Conclusions
Findings here provide further evidence that L. arcticus can act as a keystone species in Arctic and alpine water bodies, with its occurrence and availability as prey having marked impacts on the body condition, growth and maturity of resident fish species such as brown trout. Monitoring and evaluation of potential climate change, acidification, water level regulation and other anthropogenic impacts on L. arcticus populations may therefore serve as an appropriate indicator of ecosystem disturbance, with changes likely to impact fish populations. Moreover, such monitoring would provide valuable information useful for management actions aimed at sustaining the biodiversity and natural ecosystem processes in oligotrophic waterbodies and would help with the preservation of key ecosystem services such as recreational fishing and maintenance of drinking water quality. Our study suggests a high importance of early-season water levels for recruitment and availability of L. arcticus as a nutritious prey for reservoir resident brown trout. Hence, instead of compensatory fish stockings, more holistic, ecosystem-based management actions, such as altered water level regulation patterns and habitat restorations, could better improve and protect the ecological status of the unique invertebrate and fish fauna found in alpine hydropower reservoirs.
References
Aass P (1969) Crustacea, especially Lepidurus arcticus Pallas, as brown trout food in Norwegian mountain reservoirs. Inst Freshw Res Drottningholm 49:183–201
Agresti A (2002) Categorical data analysis, 2nd edn. John Wiley and Sons, Hoboken
Arnold GP (1966) Observations on Lepidurus arcticus (Pallas) (Crustacea, Notostraca) in east Greenland. Ann Mag Nat Hist 9:599–617
Bates DM, Watts DG (1988) Nonlinear regression analysis and its applications. Wiley, New York
Bonalumi M, Anselmetti FS, Wueest A, Schmid M (2012) Modeling of temperature and turbidity in a natural lake and a reservoir connected by pumped-storage operations. Water Resour Res 48:W08508
Borgstrøm R (1970) Skjoldkreps, Lepidurus arcticus, i Stolsvannsmagasinet i Hallingdal. Fauna 23:12–20 (In Norwegian with an English summary)
Borgstrøm R (1973) The effect of increased water level fluctuation upon the brown trout population of Mårvann, a Norwegian reservoir. Norw J Zool 21:101–112
Borgstrøm R (2019) Skjoldkreps – eit viktig næringsdyr i høgfjellsvatn som er sterkt påverka av miljøfaktorar. Vann 1:33–42 (In Norwegian with an English summary)
Borgstrøm R, Hendrey GR (1976) pH tolerance of the first larval stages of L. arcticus arcticus (Pallas) and adult Gammarus lacustris G.O.Sars. SNSF-prosjektet, Oslo-Ås, Norway, IR 22/76: 1–37
Borgstrøm R, Garnås E, Saltveit SJ (1985) Interactions between brown trout, Salmo trutta L., and minnow, Phoxinus phoxinus (L.) for their common prey, Lepidurus arcticus (Pallas). Verh Int Ver Theor Angew Limnol 22:2548–2552
Borgstrøm R, Brabrand Å, Solheim JT (1992) Effects of siltation on resource utilization and dynamics of allopatric brown trout, Salmo trutta, in a reservoir. Environ Biol Fishes 34:247–255
Borgstrøm R, Aas M, Hegseth H, Dempson JB, Svenning MA (2018) Lepidurus arcticus (Crustacea: Notostraca); an unexpected prey of Arctic charr (Salvelinus alpinus) in a High Arctic river. Boreal Environ Res 23:149–157
Brabrand Å (2010) Virkning av reguleringshøyde og ulik manøvrering på næringsdyr i reguleringsmagasiner. LFI Oslo Rapport nr. 281–2010. https://www.nhm.uio.no/
Breistein J, Nøst T (1997) Standardisering av måle- og biomasseberegnings-metoder for dyreplankton, bunndyr, overflateinsekter og fisk i ferskvann. NINA Oppdragsmelding 480:1–19 (In Norwegian, available at www.nina.no)
Brown LC, Duguay CR (2010) The response and role of ice cover in lake-climate interactions. Prog Phys Geogr 34:671–704
Chapman DG, Robson DS (1960) The analysis of a catch curve. Biometrics 16:354–368
Chen Y, Jackson DA, Harvey HH (1992) A comparison of von Bertalanffy and polynomial functions in modelling fish growth data. Can J Fish Aquat Sci 49:1228–1235
Christoffersen K (2001) Predation on Daphnia pulex by Lepidurus arcticus. Hydrobiologia 442:223–229
Coulson SJ, Convey P, Aakra K, Aarvik L, Ávila-Jiménez ML, Babenko A, Biersma EM, Boström S, Brittain JE, Carlsson AM, Christoffersen K, De Smet WH, Ekrem T, Fjellberg A, Füreder L, Gustafsson D, Gwiazdowicz DJ, Hansen LO, Holmstrup M, Hullé M, Kaczmarek Ł, Kolicka M, Kuklin V, Lakka H-K, Lebedeva N, Makarova O, Maraldo K, Melekhina E, Ødegaard F, Pilskog HE, Simon JC, Sohlenius B, Solhøy T, Søli G, Stur E, Tanasevitch A, Taskaeva A, Velle G, Zawierucha K, Zmudczyńska-Skarbek K (2014) The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol Biochem 68:440–470
Dararat W, Lomthaisong K, Sanoamuang L (2012) Biochemical composition of three species of fairy shrimps (Branchiopoda: Anostraca) from Thailand. J Crustacean Biol 32:81–87
Diana JS (2004) Biology and ecology of fishes, 2nd edn. Cooper Publishing Group, LLC, Traverse City
Dodrill MJ, Yackulic CB, Kennedy TA, Hayes JW (2016) Prey size and availability limits maximum size of rainbow trout in a large tailwater: insights from a drift-foraging bioenergetics model. Can J Fish Aquat Sci 58:1122–1132
Dunn A, Francis RICC, Doonan IJ (2002) Comparison of the Chapman-Robson and regression estimators of Z from catch-curve data when non-sampling stochastic error is present. Fish Res 59:149–159
Einarsson Á, Stefánsdóttir G, Jóhannesson H, Ólafsson JS, Gíslason GM, Wakana I, Gudbergsson G, Gardarsson A (2004) The ecology of Lake Myvatn and the River Laxá: variation in space and time. Aquat Ecol 38:317–348
Elliott JM (1976) The energetics of feeding, metabolism and growth of brown trout (Salmo trutta) in relation to body weight, water temperature and ration size. J Anim Ecol 45:923–948
Elliott JM (1994) Quantitative ecology and the Brown Trout. Oxford University press, Oxford
Elliott JM, Fletcher JM (2001) A comparison of three methods for assessing the abundance of Arctic charr, Salvelinus alpinus, in Windermere (northwest England). Fish Res 53:39–46
Eloranta AP, Sánchez-Hernández J, Amundsen P-A, Skoglund S, Brush JM, Henriksen EH, Power M (2017) Water level regulation affects niche use of a lake top predator, Arctic charr (Salvelinus alpinus). Ecohydrology 10:e1766
Eloranta AP, Mariash HL, Rautio M, Power M (2013) Lipid-rich zooplankton subsidise the winter diet of benthivorous Arctic charr (Salvelinus alpinus) in a subarctic lake. Freshw Biol 58:2541–2554
Eloranta AP, Finstad AF, Helland IP, Ugedal O, Power M (2018) Hydropower impacts on reservoir fish populations are modified by environmental variation. Sci Total Environ 618:313–322
Fjellheim A, Tysse Å, Bjerknes V (2001) Reappearance of highly acid-sensitive invertebrates after liming of an alpine lake ecosystem. Water Air Soil Pollut 130:1391–1396
Fjellheim A, Tysse Å, Bjerknes V (2007) Fish stomachs as a biomonitoring tool in studies of invertebrate recovery. Water Air Soil Pollut 7:293–300
Gebre S, Alfredsen K, Lia L, Stickler M, Tesaker E (2013) Review of ice effects on hydropower systems. J Cold Reg Eng 27:196–222
Haddon M (2001) Modelling and quantitative methods in fisheries. Chapman and Hall/CRC, Boca Raton
Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18
Haugen TO, Rygg TA (1996a) Food and habitat-segregation in sympatric grayling and brown trout. J Fish Biol 49:301–318
Haugen TO, Rygg TA (1996b) Intra- and inter-specific life history differences in sympatric grayling and brown trout in a Norwegian reservoir. J Fish Biol 48:964–978
Heggen MP, Birks HH, Anderson NJ (2010) Long-term ecosystem dynamics of a small lake and its catchment in west Greenland. Holocene 20:1207–1222
Hesthagen T (2018) Fangstutbytet og bestandstilhøva hjå auren i Aursjoen-magasinet i Skjåk kommune i søraustlege delen av Reinheimen i åra 1980–2017. NINA Rapport 1446 (In Norwegian, available at www.nina.no)
Hesthagen T, Hegge O, Skurdal J (1992) Food choice and vertical distribution of European minnow, Phoxinus phoxinus, and young native and stocked brown trout, Salmo trutta, in the littoral zone of a subalpine lake. Nord J Freshw Res 67:72–76
Hirsch PE, Eloranta AP, Amundsen P-A, Brabrand Å, Charmasson J, Helland IP, Power M, Sánchez-Hernández J, Sandlund OT, Sauterleute JF, Skoglund S, Ugedal O, Yang H (2017) Effects of water level regulation in alpine hydropower reservoirs – an ecosystem perspective with a special emphasis on fish. Hydrobiologia 794:287–301
IPCC (Intergovernmental Panel on Climate Change), Pachauri RK, Meyer LA (eds) (2014) Climate Change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
Järvinen A, Lakka H-K, Sujala M (2014) Arktinen kilpikidusjalkainen, tunturivesien elävä fossiili, löydetty jälleen Suomesta. Luonnon Tutkija 1:19–24 (In Finnish)
Jeppesen E, Christoffersen K, Landkildehus F, Lauridsen T, Amsinck SL, Riget F, Søndergaard M (2001) Fish and crustaceans in northeast Greenland lakes with special emphasis on interactions between Arctic charr (Salvelinus alpinus), Lepidurus arcticus and benthic chydorids. Hydrobiologia 442:329–337
Jonsson B, Jonsson N (2011) Ecology of Atlantic salmon and brown trout – Habitat as a template for life histories. Springer, New York
Kerr SR (1971) Prediction of growth efficiency in nature. J Fish Res Board Can 28:809–814
Koksvik JI (1995) Seasonal occurrence and diel locomotor activity in littoral Cladocera in a mesohumic lake in Norway. Hydrobiologia 307:193–201
Kristensen DM, Jørgensen TR, Larsen RK, Forchhammer MC, Christoffersen KS (2006) Inter-annual growth of Arctic charr (Salvelinus alpinus, L.) in relation to climate variation. BMC Ecol 6:10
L’Abée-Lund JH, Sægrov H (1991) Resource use, growth and effects of stocking in alpine brown trout, Salmo trutta L. Aquac Res 22:519–526
Lakka H-K (2013) The ecology of a freshwater crustacean: Lepidurus arcticus (Brachiopoda; Notostraca) in a High Arctic region. MSc thesis no. 111, University of Helsinki, Lahti, Finland
Lakka H-K, Leppänen A, Mykrä H, Vaajala M, Raineva S, Lensu T, Salonen E (2019) Erittäin uhanalainen paljakkakilpiäinen on siian ravintoa Inarijärvessä. Luonnon Tutkija 2:66–70 (In Finnish)
Lento J, Goedkoop Q, Culp J, Christoffersen KS, Lárusson KF, Fefilova E, Guðbergsson G, Liljaniemi P, Ólafsson JS, Sandøy S, Zimmerman C, Christensen T, Chambers P, Heino J, Hellsten S, Kahlert M, Keck F, Laske S, Chun Pong Lau D, Lavoie I, Levenstein B, Mariash H, Rühland K, Saulnier-Talbot E, Schartau AK, Svenning M (2019) State of the Arctic Freshwater Biodiversity. Conservation of Arctic Flora and Fauna International Secretariat, Akureyri, Iceland. ISBN 978-9935-431-77-6
Lien L (1978) The energy budget of the brown trout population of Øvre Heimdalsvatn. Holarctic Ecol 1:279–300
Lindholm M, Stordal F, Moe SJ, Hessen DG, Aass P (2012) Climate-driven range retraction of an Arctic freshwater crustacean. Freshw Biol 57:2591–2601
Longhurst AR (1955) A review of the Notostraca. Bull Br Mus Nat Hist Zool 3:1–57
MacNeil C, Elwood RW, Dick JTA (1999) Predator–prey interactions between brown trout Salmo trutta and native and introduced amphipods; their implications for fish diets. Ecography 22:686–696
Mittelbach GG, Persson L (1998) The ontogeny of piscivory and its ecological consequences. Can J Fish Aquat Sci 55:1454–1465
Moulton LL, Morris WA, George C, Rose JR, Whitman M (2007) Surveys of fish habitat in the Teshekpuk Lake region, 2003–2005. Department of Wildlife Management, North Slope Borough
Pasquali V, Calizza E, Setini A, Hazlerigg D, Christoffersen KS (2019) Preliminary observations on the effect of light and temperature on the hatching success and rate of Lepidurus arcticus eggs. Ethol Ecol Evol 31:348–357
Primicerio R, Klemetsen A (1999) Zooplankton seasonal dynamics in the neighbouring lakes Takvatn and Lombola (Northern Norway). Hydrobiologia 411:19–29
Quakenbush L, Suydam R, Obritschkewitsch T, Deering M (2004) Breeding biology of Steller's Eiders (Polysticta stelleri) near Barrow, Alaska, 1991–99. Arctic 57:166–182
Qvenild T, Hesthagen T (2019) Environmental conditions limit the distribution of Lepidurus arcticus (Branchiopoda: Notostraca) in lakes on the Hardangervidda mountain plateau, Southern Norway. Fauna Norv 39:77–110
Qvenild T, Hesthagen T (2020) The viability of the crustacean Eurycercus lamellatus (Branciopoda, Cladocera) in a high mountain area of southern Norway. Fauna Norv 40:22–42
Qvenild T, Fjeld E, Fjellheim A, Rognerud S, Tysse Å (2018) Climatic effects on a cold stenotherm species Lepidurus arcticus (Branchiopoda, Notostraca) on the southern outreach of its distribution range. Fauna Norv 38:37–53
Rautio M, Dufresne F, Laurion I, Bonilla S, Vincent W, Christoffersen KS (2011) Shallow freshwater ecosystems of the circumpolar Arctic. Ecoscience 18:204–222
Reznick D, Bryant MJ, Bashley F (2002) r- and K-selection revisited: the role of population regulation in life-history evolution. Ecology 83:1509–1520
Ringler NH (1979) Selective predation by drift-feeding brown trout Salmo trutta. J Fish Res Board Can 36:392–403
Robson DS, Chapman DG (1961) Catch curves and mortality rates. Trans Am Fish Soc 90:181–189
Rogers DC (2001) Revision of the Nearctic L. arcticus (Notostraca). J Crust Biol 21:991–1006
Rogers DC (2014) Larger hatching fractions in avian dispersed anostracan eggs (Branchiopoda). J Crust Biol 34:135–143
Sægrov H, Hellen BA, Kambestad M (2013) Fiskeundersøkelser i Namsvatnet i Røyrvik i 2013. Rapport nr. 1885, 52p. ISBN 978-82-8308-071-1
Sánchez-Hernández J, Cobo F (2015) Adaptive flexibility in the feeding behaviour of brown trout: optimal prey size. Zool Stud 54:26
Smirnov NN (1962) Eurycercus lamellatus (O. F. Müller) (Chydoridae, Cladocera): field observations and nutrition. Hydrobiologia 20:280–294
Smith EP (2002) BACI design. In: El-Shaarawi AH, Piegorsch WW (eds) Encyclopedia of environmetrics. Wiley, Chichester, pp 141–148
Sømme S (1934) Contributions to the Biology of Norwegian fish food animals. I. Lepidurus arcticus. Avhandl Norske Vidensk Akad 6:1–36
Stander E (2006) Ice stresses in reservoirs: effect of water level fluctuations. J Cold Reg Eng 20:52–67
Stuart-Smith RD, Richardson AMM, White RWG (2004) Increasing turbidity significantly alters the diet of brown trout: a multi-year longitudinal study. J Fish Biol 65:376–388
Väinölä R, Könönen K, Lakka H-K, Uddström A, Mannerkoski I, Erkamo E, Arponen H, Keskinen E, Lanki M, Laine AO (2019) Crustaceans Crustacea. In: Hyvärinen E, Juslén A, Kemppainen E, Uddström A, Liukko U-M (eds) Suomen lajien uhanalaisuus Punainen kirja 2019 (The 2019 Red List of Finnish Species). Ministry of the Environment & Finnish Environment Institute, Helsinki, pp 344–348
Vincent WF (2009) Effects of climate change on lakes. In: Likens GE (ed) Encyclopedia of inland waters. Academic Press, Cambridge, pp 55–60
Wankowski JWJ, Thorpe JE (1979) The role of food particle size in the growth of juvenile Atlantic salmon (Salmo salar L.). J Fish Biol 14:351–370
Ware DM (1972) Predation by rainbow trout (Salmo gairdneri): the influence of hunger, prey density, and prey size. J Fish Res Board Can 29:1193–1201
Weidman RP, Schindler DW, Vinebrooke RD (2011) Pelagic food web interactions among benthic invertebrates and trout in mountain lakes. Freshw Biol 56:1081–1094
Westling A (ed) (2015) Rödlistade arter i Sverige 2015. ArtDatabanken SLU, Uppsala
Wetterer JK (1989) Mechanisms of prey choice by planktivorous fish: perceptual constraints and rules of thumb. Anim Behav 37:955–967
Woods PJ, Skúlason S, Snorrason SS, Kristjánsson BK, Ingimarsson F, Malmquist HJ (2013) Variability in the functional role of Arctic charr Salvelinus alpinus as it relates to lake ecosystem characteristics. Environ Biol Fish 96:1361–1376
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice Hall, Upper Saddle River
Acknowledgements
We want to thank all the people who have helped with fieldwork during the long-term monitoring in Aursjoen. We also thank the three anonymous reviewers for their constructive feedback on an earlier draft of the manuscript. The study has received funding from the Environmental Division of the former County of Oppland, from the Department of Biological and Environmental Science at the University of Jyväskylä, and from the Research Council of Norway (project # 228714/E20).
Funding
Open access funding provided by University of Jyväskylä (JYU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lakka, HK., Eloranta, A.P., Hesthagen, T. et al. Impacts of reduced Lepidurus arcticus availability on brown trout life history traits in a mountain reservoir. Aquat Sci 82, 78 (2020). https://doi.org/10.1007/s00027-020-00751-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00751-x