Abstract
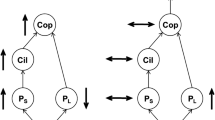
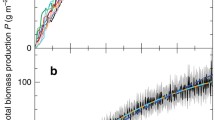
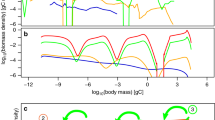
Understanding variation in trophic cascades is critical for developing management strategies for invasive and introduced species as well as in designing biomanipulation experiments. We evaluated, in fish driven trophic cascades, the responses of invertebrate biomass, body size, and production both experimentally and through a model. Experimental mesocosm treatments of small (Moina), large (Daphnia pulex), and mixed size zooplankton communities were characterized with and without size-selective fish (bluegill, Lepomis macrochirus). Experimental responses were typical of trophic cascades showing reduced biomass, body size and production of zooplankton and generally enhanced chlorophyll a. Although zooplankton biomass was similarly reduced in all fish treatments, production for the fish D. pulex treatment was lower than the other fish treatments because larger taxa have a lower ratio of production to biomass (P/B). As would be expected based on zooplankton production among fish treatments, response of chlorophyll a in treatments with D. pulex was faster and final concentration higher than in Moina or mixed size treatments. A model of changes in production under a range of reductions in body size and/or biomass, from no reduction to 99% reduction, supported experimental results indicating invertebrate production can be resilient to reduction of biomass when body size is reduced. Strength of trophic cascades may therefore, in part, be related to interactions among reduction in biomass, size-selective predation, and size frequency distribution and plasticity of prey community. Because size-selective predation is a common trait in trophic cascades, some level of compensation is likely in most trophic cascades.



Similar content being viewed by others
Data accessibility
Data are available through the citation: Detmer, Thomas; Wahl, David (2019): Trophic cascade strength is influenced by size frequency distribution of primary consumers and size-selective predation: examined with mesocosms and modeling. University of Illinois at Urbana-Champaign. https://doi.org/10.13012/B2IDB-3292716_V1.
References
Baumgartner D, Rothhaupt KO (2003) Predictive length-dry mass regressions for freshwater invertebrates in a pre-alpine lake littoral. Int Rev Hydrobiol 88:453–463
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc 18:308–343
Betsill RK, van den Avyle MJ (1994) Spatial heterogeneity of reservoir zooplankton: a matter of timing? Hydrobiologia 277:63–70
Brett MT, Goldman CR (1996) A meta-analysis of the freshwater trophic cascade. Proc Natl Acad Sci USA 93:7723–7726
Brooks JL, Dodson SI (1965) Predation body size and composition of plankton. Science 150:28–35
Brown JH, Marquet PA, Taper ML (1993) Evolution of body-size: consequences of an energetic definition of fitness. Am Nat 142:573–584
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Cáceres CE, Soluk DA (2002) Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131:402–408
Carey MP, Wahl DH (2010) Native fish diversity alters the effects of an invasive species on food webs. Ecology 91:2965–2974
Carlisle DM, Hawkins CP (1998) Relationships between invertebrate assemblage structure, 2 trout species, and habitat structure in Utah mountain lakes. J N Am Benthol Soc 17:286–300
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639
Carpenter SR et al (1987) Regulation of lake primary productivity by food web structure. Ecology 68:1863–1876
Collins SF, Detmer TM, Nelson KA, Nannini MA, Sass GG, Wahl DH (2018) The release and regulation of rotifers: examining the predatory effects of invasive juvenile common and bighead carp. Hydrobiologia 813:199–211
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813
Demi LM, Simon KS, Coghlan SM, Saunders R, Anderson D (2012) Anadromous alewives in linked lake-stream ecosystems: do trophic interactions in lakes influence stream invertebrate communities? Freshw Sci 31:973–985
DeMott WR, Kerfoot WC (1982) Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecology 63:1949–1966
Detmer TM (2019) Zooplankton nearshore compared to offshore in historically fishless lakes of high elevation are influenced by the introduction of planktivorous fish and water residence time. J Plankton Res. https://doi.org/10.1093/plankt/fbz005
Detmer TM, McCutchan JH, Lewis WM (2017a) Predator driven changes in prey size distribution stabilize secondary production in lacustrine food webs. Limnol Oceanogr 62:592–605
Detmer TM, McCutchan JH, Lewis WM (2017b) Trophic interactions across lake-stream boundaries in mountain lakes. Inland Waters 7:440–448
Detmer TM, Diana MJ, Wahl DH (2019) Season and presence of Gizzard Shad influence horizontal spatial distribution of zooplankton in reservoirs of the midwestern United States. Freshw Sci. https://doi.org/10.1086/701676
DeVries DR, Stein RA (1992) Complex interactions between fish and zooplankton: quantifying the role of an open-water planktivore. Can J Fish Aquat Sci 49:1216–1227
Drenner RW, Baca RM, Gilroy JS, Ernst MR, Jensen DJ, Marshall DH (2002) Community responses to piscivorous largemouth bass: a biomanipulation experiment. Lake Reserv Manage 18:44–51
Dumont HJ, Balvay G (1979) Dry-weight estimate of Chaoborus flavicans (meigen) as a function of length and instars. Hydrobiologia 64:139–145
Dumont HJ, Van de Velde I, Dumont S (1975) The dry weight estimate of biomass in a selection of cladocera, copepod, and rotifer from the plankton, periphyton and benthos of continental waters. Oecologia 19:75–97
EPA Great Lakes National Office (2003) Standard operating procedure for zooplankton analysis. Environmental Protection Agency, U.S
Hildrew AG, Raffaelli DG, Edmonds-Brown R (2007) Body size: the structure and function of aquatic ecosystems. Cambridge University Press, Cambridge
Jeppesen E, Jensen JP, Sondergaard M, Lauridsen T (1999) Trophic dynamics in turbid and clearwater lakes with special emphasis on the role of zooplankton for water clarity. Hydrobiologia 408:217–231
Jeppesen E, Lauridsen TL, Mitchell SF, Christoffersen K, Burns CW (2000) Trophic structure in the pelagial of 25 shallow New Zealand lakes: changes along nutrient and fish gradients. J Plankton Res 22:951–968
Johnston TA, Cunjak RA (1999) Dry mass-length relationships for benthic insects: a review with new data from Catamaran Brook, New Brunswick, Canada. Freshw Biol 41:653–674
Kerr SR, Dickie LM (2001) The biomass spectrum: a predator–prey theory of aquatic production. Columbia University Press, New York
Knapp RA, Corn PS, Schindler DE (2001) The introduction of nonnative fish into wilderness lakes: Good intentions, conflicting mandates, and unintended consequences. Ecosystems 4:275–278
Kratina P, Greig HS, Thompson PL, Carvalho-Pereira TSA, Shurin JB (2012) Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 93:1421–1430
Lynch M, Shapiro J (1981) Predation, enrichment, and phytoplankton community structure. Limnol Oceanogr 26:86–102
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798
McNaught AS, Schindler DW, Parker BR, Paul AJ, Anderson RS, Donald DB, Agbeti M (1999) Restoration of the food web of an alpine lake following fish stocking. Limnol Oceanogr 44:127–136
McQueen DJ, Post JR (1988) cascading trophic interactions—uncoupling at the zooplankton-phytoplankton link. Hydrobiologia 159:277–296
Mehner T, Benndorf J, Kasprzak P, Koschel R (2002) Biomanipulation of lake ecosystems: successful applications and expanding complexity in the underlying science. Freshw Biol 47:2453–2465
Merritt RW, Cummins KW, Berg MB (eds) (2008) An introduction to aquatic insects of North America, 4th edn. Kendall/Hunt Publishing Company, Dubuque
Mittelbach GG (1981) Foraging efficiency and body size—a study of optimal diet and habitat use by bluegills. Ecology 62:1370–1386
Pagano M (2008) Feeding of tropical cladocerans (Moina micrura, Diaphanosoma excisum) and rotifer (Brachionus calyciflorus) on natural phytoplankton: effect of phytoplankton size–structure. J Plankton Res 30:401–414
Parker BR, Schindler DW (2006) Cascading trophic interactions in an oligotrophic species-poor alpine lake. Ecosystems 9:157–166
Parker BR, Schindler DW, Donald DB, Anderson RS (2001) The effects of stocking and removal of a nonnative salmonid on plankton of an alpine lake. Ecosystems 4:334–345
Pennak RW (1989) Fresh-water invertebrates of the United States: Protozoa to Mollusca. Wiley, New York
Peters RH (1986) The ecological implications of body size, vol 2. Cambridge University Press, Cambridge
Plante C, Downing JA (1989) Production of fresh-water invertebrate populations in lakes. Can J Fish Aquat Sci 46:1489–1498
Polis GA (1999) Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86:3–15
Porter RH (1973) Selective grazing and differential digestion of algae by zooplankton. Nature 224:179–180
Power ME (1990) Effects of fish in river food webs. Science 250:811–814
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. http://www.R-project.org/
Sarnelle O (1992) Nutrient enrichment and grazer effects on phytoplankton in lakes. Ecology 73:551–560
Sheldon RW (1972) Size separation of marine seston by membrane and glass-fiber filters. Limnol Oceanogr 17:494–498
Shurin JB (2001) Interactive effects of predation and dispersal on zooplankton communities. Ecology 82:3404–3416
Sprules WG (1972) Effects of size-selective predation and food competition on high-altitude zooplankton communities. Ecology 53:375–386
Stein RA, DeVries DR, Dettmers JM (1995) Food-web regulation by a planktivore: exploring the generality of the trophic cascade hypothesis. Can J Fish Aquat Sci 52:2518–2526
Stevenson CF, Demes KW, Salomon AK (2016) Accounting for size-specific predation improves our ability to predict the strength of a trophic cascade. Ecol Evol 6:1041–1053
Strock KE, Saros JE, Simon KS, McGowan S, Kinnison MT (2013) Cascading effects of generalist fish introduction in oligotrophic lakes. Hydrobiologia 711:99–113
Symons CC, Shurin JB (2016) Climate constrains lake community and ecosystem responses to introduced predators. P R Soc B 283:20160825
Thorp JH, Covich AP (1991) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego
van Donk E, Hessen D (1993) Grazing resistance in nutrient-stressed phytoplankton. Oecologia 93:508–511
Vanni MJ, Findlay DL (1990) Trophic cascades and phytoplankton community structure. Ecology 71:921–937
Warfe DM, Barmuta LA (2006) Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 150:141–154
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42:487–495
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll-a in the presence of chlorophyll-b and pheopigments. Limnol Oceanogr 39:1985–1992
Werner EE, Hall DJ (1974) Optimal foraging and size selection of prey by bluegill sunfish (lepomis-macrochirus). Ecology 55:1042–1052
Wetzel RG, Likens G (2000) Limnological Analyses, 2nd edn. Springer, New York
Wicklum D (1999) Variation in horizontal abundance in mountain lakes: shore avoidance or fish predation? J Plankton Res 21:1957–1975
Acknowledgements
We would like to thank the Kaskaskia Biological Station technicians for their assistance in collecting and processing samples. In addition, we are grateful to the two anonymous reviewers for providing helpful suggestions for an early draft of this study.
Author information
Authors and Affiliations
Contributions
TMD and DHW conceived and designed the experiments. TMD and DHW performed the experiments. TMD and DHW analyzed the data. TMD and DHW wrote the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Detmer, T.M., Wahl, D.H. Trophic cascade strength is influenced by size frequency distribution of primary consumers and size-selective predation: examined with mesocosms and modeling. Aquat Sci 81, 52 (2019). https://doi.org/10.1007/s00027-019-0648-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-019-0648-x