Abstract.
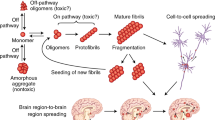
Recent findings strongly support the hypothesis that diverse human disorders, including the most common neurodegenerative diseases, arise from misfolding and aggregation of an underlying protein. Despite the good evidence for the involvement of protein misfolding in disease pathogenesis, the mechanism by which protein conformational changes participate in the disease is still unclear. Among the best-studied diseases of this group are the transmissible spongiform encephalopathies or prion-related disorders, in which misfolding of the normal prion protein plays a key role in the disease. In this article we review recent data on the link between prion protein misfolding and the pathogensis of spongiform encephalopathies.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 15 July 2002; received after revision 19 August 2002; accepted 23 August 2002
RID="*"
ID="*"Corresponding author.
Rights and permissions
About this article
Cite this article
Hetz, C., Soto, C. Protein misfolding and disease: the case of prion disorders. CMLS, Cell. Mol. Life Sci. 60, 133–143 (2003). https://doi.org/10.1007/s000180300009
Issue Date:
DOI: https://doi.org/10.1007/s000180300009