Abstract.
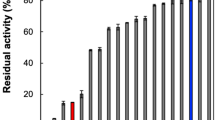
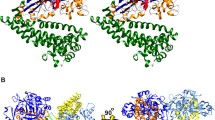
The study of the interactions between the Tyr280Phe mutant of the Streptomyces R61 DD-peptidase, various substrates and β-lactam antibiotics shows that Tyr280 is involved not only in the formation of the acylenzyme with the peptide substrate and β-lactam antibiotics, but also and specifically in the catalysis of the transpeptidation reaction. Surprisingly, this residue does not belong to the conserved structural and functional elements which characterise the penicillin-recognising enzymes.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 30 April 1998; accepted 5 May 1998
Rights and permissions
About this article
Cite this article
Wilkin, JM., Lamotte-Brasseur, J. & Frère, JM. The catalytic mechanism of DD-peptidases: unexpected importance of tyrosine 280 in the transpeptidation reaction catalysed by the Streptomyces R61 DD-peptidase. CMLS, Cell. Mol. Life Sci. 54, 726–732 (1998). https://doi.org/10.1007/s000180050200
Issue Date:
DOI: https://doi.org/10.1007/s000180050200