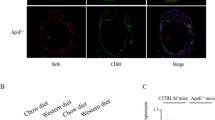
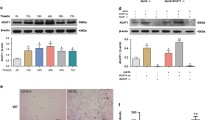
Abstract
Atherosclerosis (AS) is a serious cardiovascular disease. One of its hallmarks is hyperlipidemia. Inhibiting the formation of macrophage foam cells is critical for alleviating AS. Transcription factor EB (TFEB) can limit the formation of macrophage foam cells by upregulating lysosomal activity. We examined whether TFEB SUMOylation is involved in this progress during AS. In this study, we investigated the role of TFEB SUMOylation in macrophages in AS using TFEB SUMOylation deficiency Ldlr−/− (TFEB-KR: Ldlr−/−) transgenic mice and TFEB-KR bone marrow-derived macrophages. We observed that TFEB-KR: Ldlr−/− atherosclerotic mice had thinner plaques and macrophages with higher lysosomal activity when compared to WT: Ldlr−/− mice. TFEB SUMOylation in macrophages decreased after oxidized low-density lipoprotein (OxLDL) treatment in vitro. Compared with wild type macrophages, TFEB-KR macrophages exhibited less lipid deposition after OxLDL treatment. Our study demonstrated that in AS, deSUMOylation of TFEB could inhibit the formation of macrophage foam cells through enhancing lysosomal biogenesis and autophagy, further reducing the accumulation of lipids in macrophages, and ultimately alleviating the development of AS. Thus, TFEB SUMOylation can be a switch to modulate macrophage foam cells formation and used as a potential target for AS therapy.









Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the paper. Data will be made available on reasonable request, not applicable for material.
Abbreviations
- AS:
-
Atherosclerosis
- TFEB:
-
Transcription factor EB
- SUMOs:
-
Small ubiquitin-like modifiers
- SENPs:
-
SUMO specific peptidases
- OxLDL:
-
Oxidized low-density lipoprotein
- IL-1β:
-
Interleukin-1β
- LAMP1:
-
Lysosomal associated membrane protein 1
- CLCN7:
-
Chloride channel, voltage-sensitive 7
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- IL-4:
-
Interleukin-4
- IL-6:
-
Interleukin-6
- CTSB:
-
Cathepsin B
- CTSD:
-
Cathepsin D
- CD36:
-
Cluster of differentiation 36
- Scarb1:
-
Scavenger receptor class B member 1
- Msr1:
-
Macrophages scavenger receptor 1
- Abcg1:
-
ATP binding cassette subfamily G member 1
- CQ:
-
Chloroquine
- Mcoln-1:
-
Mucolipin-1
- Trpml1:
-
Transient receptor potential channel mucolipin-1
- FACT:
-
Facilitates chromatin transcription
- SSRP1:
-
Structure specific recognition protein 1
- SUPT16H:
-
SPT16 homolog, facilitates chromatin remodeling subunit
- NaAsO2 :
-
Sodium arsenite
References
Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ (2017) Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol 16:s27–s42
Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Maatta A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Yla-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL (2018) Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558:301–306
Tontonoz P, Wu X, Jones M, Zhang Z, Salisbury D, Sallam T (2017) Long noncoding RNA facilitated gene therapy reduces atherosclerosis in a murine model of familial hypercholesterolemia. Circulation 136:776–778
Bjorkegren JLM, Lusis AJ (2022) Atherosclerosis: recent developments. Cell 185:1630–1645
Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B (2017) Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun 8:15750
Edgar L, Akbar N, Braithwaite AT, Krausgruber T, Gallart-Ayala H, Bailey J, Corbin AL, Khoyratty TE, Chai JT, Alkhalil M, Rendeiro AF, Ziberna K, Arya R, Cahill TJ, Bock C, Laurencikiene J, Crabtree MJ, Lemieux ME, Riksen NP, Netea MG, Wheelock CE, Channon KM, Ryden M, Udalova IA, Carnicer R, Choudhury RP (2021) Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144:961–982
Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL (2011) Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 13:655–667
Robichaud S, Fairman G, Vijithakumar V, Mak E, Cook DP, Pelletier AR, Huard S, Vanderhyden BC, Figeys D, Lavallee-Adam M, Baetz K, Ouimet M (2021) Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy 17:3671–3689
Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533
Zhao X (2018) SUMO-mediated regulation of nuclear functions and signaling processes. Mol Cell 71:409–418
Ma G, Li S, Han Y, Li S, Yue T, Wang B, Jiang J (2016) Regulation of smoothened trafficking and hedgehog signaling by the SUMO pathway. Dev Cell 39:438–451
Conti L, Nelis S, Zhang C, Woodcock A, Swarup R, Galbiati M, Tonelli C, Napier R, Hedden P, Bennett M, Sadanandom A (2014) Small ubiquitin-like modifier protein SUMO enables plants to control growth independently of the phytohormone gibberellin. Dev Cell 28:102–110
Wilson VG (2017) Introduction to sumoylation. Adv Exp Med Biol 963:1–12
Yang M, Liu E, Tang L, Lei Y, Sun X, Hu J, Dong H, Yang SM, Gao M, Tang B (2018) Emerging roles and regulation of MiT/TFE transcriptional factors. Cell Commun Signal 16:31
Tol MJ, van der Lienden MJC, Gabriel TL, Hagen JJ, Scheij S, Veenendaal T, Klumperman J, Donker-Koopman WE, Verhoeven AJ, Overkleeft H, Aerts JM, Argmann CA, van Eijk M (2018) HEPES activates a MiT/TFE-dependent lysosomal-autophagic gene network in cultured cells: a call for caution. Autophagy 14:437–449
Puertollano R, Ferguson SM, Brugarolas J, Ballabio A (2018) The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J 37:e98804
Tao H, Yancey PG, Blakemore JL, Zhang Y, Ding L, Jerome WG, Brown JD, Vickers KC, Linton MF (2021) Macrophage SR-BI modulates autophagy via VPS34 complex and PPARalpha transcription of Tfeb in atherosclerosis. J Clin Investig 131:e94229
Fang S, Wan X, Zou X, Sun S, Hao X, Liang C, Zhang Z, Zhang F, Sun B, Li H, Yu B (2021) Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis 12:88
Jeong SJ, Stitham J, Evans TD, Zhang X, Rodriguez-Velez A, Yeh YS, Tao J, Takabatake K, Epelman S, Lodhi IJ, Schilling JD, DeBosch BJ, Diwan A, Razani B (2021) Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 17:3740–3752
Chen Z, Ouyang C, Zhang H, Gu Y, Deng Y, Du C, Cui C, Li S, Wang W, Kong W, Chen J, Cai J, Geng B (2022) Vascular smooth muscle cell-derived hydrogen sulfide promotes atherosclerotic plaque stability via TFEB (transcription factor EB)-mediated autophagy. Autophagy 18:2270–2287
Li M, Wang Z, Wang P, Li H, Yang L (2021) TFEB: a emerging regulator in lipid homeostasis for atherosclerosis. Front Physiol 12:639920
Zhao J, Hu B, Xiao H, Yang Q, Cao Q, Li X, Zhang Q, Ji A, Song S (2021) Fucoidan reduces lipid accumulation by promoting foam cell autophagy via TFEB. Carbohydr Polym 268:118247
Miller AJ, Levy C, Davis IJ, Razin E, Fisher DE (2005) Sumoylation of MITF and its related family members TFE3 and TFEB. J Biol Chem 280:146–155
Qiu S, Liang Z, Wu Q, Wang M, Yang M, Chen C, Zheng H, Zhu Z, Li L, Yang G (2022) Hepatic lipid accumulation induced by a high-fat diet is regulated by Nrf2 through multiple pathways. FASEB J 36:e22280
Endo-Umeda K, Nakashima H, Komine-Aizawa S, Umeda N, Seki S, Makishima M (2018) Liver X receptors regulate hepatic F4/80 (+) CD11b(+) Kupffer cells/macrophages and innate immune responses in mice. Sci Rep 8:9281
Wang J, Sjoberg S, Tia V, Secco B, Chen H, Yang M, Sukhova GK, Shi GP (2013) Pharmaceutical stabilization of mast cells attenuates experimental atherogenesis in low-density lipoprotein receptor-deficient mice. Atherosclerosis 229:304–309
Breder I, Coope A, Arruda AP, Razolli D, Milanski M, DorighelloGde G, de Oliveira HC, Velloso LA (2010) Reduction of endoplasmic reticulum stress—a novel mechanism of action of statins in the protection against atherosclerosis. Atherosclerosis 212:30–31
Unuma K, Aki T, Funakoshi T, Hashimoto K, Uemura K (2015) Extrusion of mitochondrial contents from lipopolysaccharide-stimulated cells: involvement of autophagy. Autophagy 11:1520–1536
Rusmini P, Cortese K, Crippa V, Cristofani R, Cicardi ME, Ferrari V, Vezzoli G, Tedesco B, Meroni M, Messi E, Piccolella M, Galbiati M, Garre M, Morelli E, Vaccari T, Poletti A (2019) Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 15:631–651
Ben M’barek K, Ajjaji D, Chorlay A, Vanni S, Foret L, Thiam AR (2017) ER membrane phospholipids and surface tension control cellular lipid droplet formation. Dev Cell 41:591-604 e7
Brancucci NMB, Gerdt JP, Wang C, De Niz M, Philip N, Adapa SR, Zhang M, Hitz E, Niederwieser I, Boltryk SD, Laffitte MC, Clark MA, Gruring C, Ravel D, Blancke Soares A, Demas A, Bopp S, Rubio-Ruiz B, Conejo-Garcia A, Wirth DF, Gendaszewska-Darmach E, Duraisingh MT, Adams JH, Voss TS, Waters AP, Jiang RHY, Clardy J, Marti M (2017) Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell 171:1532-1544 e15
Gayle S, Landrette S, Beeharry N, Conrad C, Hernandez M, Beckett P, Ferguson SM, Mandelkern T, Zheng M, Xu T, Rothberg J, Lichenstein H (2017) Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 129:1768–1778
Zhang J, Wang J, Zhou Z, Park JE, Wang L, Wu S, Sun X, Lu L, Wang T, Lin Q, Sze SK, Huang D, Shen HM (2018) Importance of TFEB acetylation in control of its transcriptional activity and lysosomal function in response to histone deacetylase inhibitors. Autophagy 14:1043–1059
Parousis A, Carter HN, Tran C, Erlich AT, Mesbah Moosavi ZS, Pauly M, Hood DA (2018) Contractile activity attenuates autophagy suppression and reverses mitochondrial defects in skeletal muscle cells. Autophagy 14:1886–1897
Ni Z, Gong Y, Dai X, Ding W, Wang B, Gong H, Qin L, Cheng P, Li S, Lian J, He F (2015) AU4S: a novel synthetic peptide to measure the activity of ATG4 in living cells. Autophagy 11:403–415
Zhou C, Ma K, Gao R, Mu C, Chen L, Liu Q, Luo Q, Feng D, Zhu Y, Chen Q (2017) Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res 27:184–201
Man SM, Kanneganti TD (2016) Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy 12:2504–2505
Zhou J, Wang Z, Wang X, Li X, Zhang Z, Fan B, Zhu C, Chen Z (2018) Dicot-specific ATG8-interacting ATI3 proteins interact with conserved UBAC2 proteins and play critical roles in plant stress responses. Autophagy 14:487–504
Frudd K, Burgoyne T, Burgoyne JR (2018) Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat Commun 9:95
Qiu B, Simon MC (2016) BODIPY 493/503 staining of neutral lipid droplets for microscopy and quantification by flow cytometry. Bio Protoc 6:e1912
Kothari V, Bornfeldt KE (2017) Liver Kinase B1 links macrophage metabolism sensing and atherosclerosis. Circ Res 121:1024–1026
Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354:472–477
Toledo JD, Garda HA, Cabaleiro LV, Cuellar A, Pellon-Maison M, Gonzalez-Baro MR, Gonzalez MC (2013) Apolipoprotein A-I Helsinki promotes intracellular acyl-CoA cholesterol acyltransferase (ACAT) protein accumulation. Mol Cell Biochem 377:197–205
Conrad KS, Cheng TW, Ysselstein D, Heybrock S, Hoth LR, Chrunyk BA, Am Ende CW, Krainc D, Schwake M, Saftig P, Liu S, Qiu X, Ehlers MD (2017) Lysosomal integral membrane protein-2 as a phospholipid receptor revealed by biophysical and cellular studies. Nat Commun 8:1908
Sorci-Thomas MG, Thomas MJ (2016) Microdomains, inflammation, and atherosclerosis. Circ Res 118:679–691
An D, Hao F, Zhang F, Kong W, Chun J, Xu X, Cui MZ (2017) CD14 is a key mediator of both lysophosphatidic acid and lipopolysaccharide induction of foam cell formation. J Biol Chem 292:14391–14400
Ma W, Ding H, Gong X, Liu Z, Lin Y, Zhang Z, Lin G (2015) Methyl protodioscin increases ABCA1 expression and cholesterol efflux while inhibiting gene expressions for synthesis of cholesterol and triglycerides by suppressing SREBP transcription and microRNA 33a/b levels. Atherosclerosis 239:566–570
Maulucci G, Chiarpotto M, Papi M, Samengo D, Pani G, De Spirito M (2015) Quantitative analysis of autophagic flux by confocal pH-imaging of autophagic intermediates. Autophagy 11:1905–1916
Nabar NR, Kehrl JH (2017) The transcription factor EB links cellular stress to the immune response. Yale J Biol Med 90:301–315
Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H (2016) MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7:12109
Jeong E, Martina JA, Contreras PS, Lee J, Puertollano R (2022) The FACT complex facilitates expression of lysosomal and antioxidant genes through binding to TFEB and TFE3. Autophagy 18:2333–2349
Wei B, Huang C, Liu B, Wang Y, Xia N, Fan Q, Chen GQ, Cheng J (2018) Mitotic phosphorylation of SENP3 regulates DeSUMOylation of chromosome-associated proteins and chromosome stability. Cancer Res 78:2171–2178
Xu Z, Chan HY, Lam WL, Lam KH, Lam LS, Ng TB, Au SW (2009) SUMO proteases: redox regulation and biological consequences. Antioxid Redox Signal 11:1453–1484
Nayak A, Muller S (2014) SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol 15:422
Mukhopadhyay D, Dasso M (2007) Modification in reverse: the SUMO proteases. Trends Biochem Sci 32:286–295
Li X, Zhang X, Zheng L, Kou J, Zhong Z, Jiang Y, Wang W, Dong Z, Liu Z, Han X, Li J, Tian Y, Zhao Y, Yang L (2016) Hypericin-mediated sonodynamic therapy induces autophagy and decreases lipids in THP-1 macrophage by promoting ROS-dependent nuclear translocation of TFEB. Cell Death Dis 7:e2527
Acknowledgements
We thank Prof. Jinke Cheng for his excellent technical assistance. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript. No sources of financial support for the conduct of the research and/or preparation of the article. No conflicts of interest to disclosure.
Funding
This research was funded by National Natural Science Foundation of China (82002937, 82000904).
Author information
Authors and Affiliations
Contributions
RC and TT designed and conceived the project, provided the concept, contributed to the manuscript and supervised the research. KZW designed and performed experiments, analyzed and interpreted the data, and prepared the manuscript. RC and TT designed experiments and edited the manuscript. LFW, WZ and GLH assisted with BMDM preparation and performed flow cytometry. All authors analyzed and interpreted the data, revised for critical intellectual content and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The funders had no conflicts of interest in the design of the study; in the collection, analysis or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethics approval
Animal studies were approved by the Animal Care and Use Committee from Xinhua Hospital, Affiliated to Medicine School of Shanghai Jiaotong University.
Consent to participate
Not applicable.
Consent for publication
All authors have been involved in writing the manuscript and consented to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2023_4981_MOESM2_ESM.tif
Supplementary Fig. 2 A: The SUMOylation site of TFEB is conserved across different species. B: Statistical analysis of the relative expression level of LC3 II; n = 3. (TIF 11510 KB)
18_2023_4981_MOESM4_ESM.tif
Supplementary Fig. 4 Under the stimulation of OxLDL, TFEB SUMOylation was not involved in NLRP3 inflammasome signal transduction. A: TFEB was knocked down in RAW264.7 macrophages. B: TFEB was overexpressed in RAW264.7 macrophages. C: The constructed macrophages were treated with OxLDL (50 μg/ml) for 24 h and then the cells lysates were immunoblotted with anti-NLRP3, anti-Caspase-1, anti-GSDMD or anti-GAPDH antibodies. (TIF 8555 KB)
18_2023_4981_MOESM5_ESM.tif
Supplementary Fig. 5 Under OxLDL stimulation, TFEB SUMOylation did not affect the polarization of macrophages. The constructed macrophages were treated with OxLDL (50 μg/ml) for 12 h and then relative mRNA levels of M1/2 maker genes were assessed by Real-time quantitative PCR; n = 3. (TIF 1983 KB)
18_2023_4981_MOESM6_ESM.tif
Supplementary Fig. 6 Under chloroquine stimulation, TFEB SUMOylation did not affect macrophage apoptosis. The constructed macrophages were treated with chloroquine for 15 h and the apoptosis of macrophages was observed through Annexin V/PI staining; n = 3. (TIF 2787 KB)
18_2023_4981_MOESM7_ESM.tif
Supplementary Fig. 7 Under OxLDL stimulation, TFEB SUMOylation did not affect the secretion of IL-1β in macrophages. The constructed macrophages were treated with OxLDL (50 μg/ml) for 24 h and then use relevant kits to detect the content of IL-1β in the cell supernatant; n = 3. (TIF 2557 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Zhou, W., Hu, G. et al. TFEB SUMOylation in macrophages accelerates atherosclerosis by promoting the formation of foam cells through inhibiting lysosomal activity. Cell. Mol. Life Sci. 80, 358 (2023). https://doi.org/10.1007/s00018-023-04981-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04981-8