Abstract
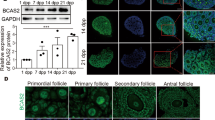
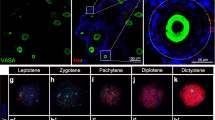
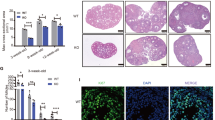
Granulosa cell abnormalities are characteristics of premature ovarian insufficiency (POI). Abnormal expression of serine/arginine-rich splicing factor 1 (SRSF1) can cause various diseases, but the role of SRSF1 in mouse granulosa cells remains largely unclear. In this study, we found that SRSF1 was expressed in the nuclei of both mouse oocytes and granulosa cells. The specific knockout of Srsf1 in granulosa cells led to follicular development inhibition, decreased granulosa cell proliferation, and increased apoptosis. Gene Ontology (GO) analysis of RNA-seq results revealed abnormal expression of genes involved in DNA repair, cell killing and other signalling pathways. Alternative splicing (AS) analysis showed that SRSF1 affected DNA damage in granulosa cells by regulating genes related to DNA repair. In summary, SRSF1 in granulosa cells controls follicular development by regulating AS of genes associated with DNA repair, thereby affecting female reproduction.







Similar content being viewed by others
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The RNA-seq data be deposited in GEO(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE228975)under accession number GSE228975.
References
Edson MA, Nagaraja AK, Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30(6):624–712. https://doi.org/10.1210/er.2009-0012
Li R, Albertini DF (2013) The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol 14(3):141–152. https://doi.org/10.1038/nrm3531
Albertini DF (1992) Regulation of meiotic maturation in the mammalian oocyte: interplay between exogenous cues and the microtubule cytoskeleton. BioEssays 14(2):97–103. https://doi.org/10.1002/bies.950140205
Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF (2000) Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 226(2):167–179. https://doi.org/10.1006/dbio.2000.9863
Meduri G, Massin N, Guibourdenche J, Bachelot A, Fiori O, Kuttenn F, Misrahi M, Touraine P (2007) Serum anti-Mullerian hormone expression in women with premature ovarian failure. Hum Reprod 22(1):117–123. https://doi.org/10.1093/humrep/del346
Chon SJ, Umair Z, Yoon MS (2021) Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol 9:672890. https://doi.org/10.3389/fcell.2021.672890
Zhang QY, Li X, Zhou XY, Li Y, Zhang J, Zhang XF, Liu YD, Chen YX, Wu XM, Ma LZ, Chen X, Chen SL (2022) Study of differential proteomics in granulosa cells of premature ovarian insufficiency (POI) and the roles and mechanism of RAC1 in granulosa cells. Mol Cell Endocrinol 555:111719. https://doi.org/10.1016/j.mce.2022.111719
Luan Y, Xu P, Yu SY, Kim SY (2021) The role of mutant p63 in female fertility. Int J Mol Sci. https://doi.org/10.3390/ijms22168968
Zhe J, Chen S, Chen X, Liu Y, Li Y, Zhou X, Zhang J (2019) A novel heterozygous splice-altering mutation in HFM1 may be a cause of premature ovarian insufficiency. J Ovarian Res 12(1):61. https://doi.org/10.1186/s13048-019-0537-x
Ghosh G, Adams JA (2011) Phosphorylation mechanism and structure of serine-arginine protein kinases. FEBS J 278(4):587–597. https://doi.org/10.1111/j.1742-4658.2010.07992.x
Cho S, Hoang A, Chakrabarti S, Huynh N, Huang DB, Ghosh G (2011) The SRSF1 linker induces semi-conservative ESE binding by cooperating with the RRMs. Nucleic Acids Res 39(21):9413–9421. https://doi.org/10.1093/nar/gkr663
Cho S, Hoang A, Sinha R, Zhong XY, Fu XD, Krainer AR, Ghosh G (2011) Interaction between the RNA binding domains of Ser-Arg splicing factor 1 and U1–70K snRNP protein determines early spliceosome assembly. Proc Natl Acad Sci U S A 108(20):8233–8238. https://doi.org/10.1073/pnas.1017700108
Das S, Krainer AR (2014) Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res 12(9):1195–1204. https://doi.org/10.1158/1541-7786.MCR-14-0131
Anczukow O, Akerman M, Clery A, Wu J, Shen C, Shirole NH, Raimer A, Sun S, Jensen MA, Hua Y, Allain FH, Krainer AR (2015) SRSF1-regulated alternative splicing in breast cancer. Mol Cell 60(1):105–117. https://doi.org/10.1016/j.molcel.2015.09.005
Anczukow O, Rosenberg AZ, Akerman M, Das S, Zhan L, Karni R, Muthuswamy SK, Krainer AR (2012) The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol 19(2):220–228. https://doi.org/10.1038/nsmb.2207
Paz S, Ritchie A, Mauer C, Caputi M (2021) The RNA binding protein SRSF1 is a master switch of gene expression and regulation in the immune system. Cytokine Growth Factor Rev 57:19–26. https://doi.org/10.1016/j.cytogfr.2020.10.008
Katsuyama T, Moulton VR (2021) Splicing factor SRSF1 is indispensable for regulatory T cell homeostasis and function. Cell Rep 36(1):109339. https://doi.org/10.1016/j.celrep.2021.109339
Ye Y, Yu F, Li Z, Xie Y, Yu X (2021) RNA binding protein serine/arginine splicing factor 1 promotes the proliferation, migration and invasion of hepatocellular carcinoma by interacting with RecQ protein-like 4 mRNA. Bioengineered 12(1):6144–6154. https://doi.org/10.1080/21655979.2021.1972785
Sun L, Lv Z, Chen X, Wang C, Lv P, Yan L, Tian S, Xie X, Yao X, Liu J, Wang Z, Luo H, Cui S, Liu J (2023) SRSF1 regulates primordial follicle formation and number determination during meiotic prophase I. BMC Biol 21(1):49. https://doi.org/10.1186/s12915-023-01549-7
Wang H, Zhang Y, Zhang J, Du X, Li Q, Pan Z (2022) circSLC41A1 resists porcine granulosa cell apoptosis and follicular atresia by promoting SRSF1 through miR-9820–5p sponging. Int J Mol Sci. https://doi.org/10.3390/ijms23031509
Xu X, Yang D, Ding JH, Wang W, Chu PH, Dalton ND, Wang HY, Bermingham JR Jr, Ye Z, Liu F, Rosenfeld MG, Manley JL, Ross J Jr, Chen J, Xiao RP, Cheng H, Fu XD (2005) ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 120(1):59–72. https://doi.org/10.1016/j.cell.2004.11.036
Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K (2014) Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet 23(4):920–928. https://doi.org/10.1093/hmg/ddt486
Hoage TR, Cameron IL (1976) Folliculogenesis in the ovary of the mature mouse: a radioautographic study. Anat Rec 184(4):699–709. https://doi.org/10.1002/ar.1091840409
Liu HX, Zhang M, Krainer AR (1998) Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins. Genes Dev 12(13):1998–2012. https://doi.org/10.1101/gad.12.13.1998
Krainer AR, Conway GC, Kozak D (1990) The essential pre-mRNA splicing factor SF2 influences 5´ splice site selection by activating proximal sites. Cell 62(1):35–42. https://doi.org/10.1016/0092-8674(90)90237-9
Gratzner HG, Leif RC, Ingram DJ, Castro A (1975) The use of antibody specific for bromodeoxyuridine for the immunofluorescent determination of DNA replication in single cells and chromosomes. Exp Cell Res 95(1):88–94. https://doi.org/10.1016/0014-4827(75)90612-6
Guo YL, Chen ZC, Li N, Tian CJ, Cheng DJ, Tang XY, Zhang LX, Zhang XY (2022) SRSF1 promotes ASMC proliferation in asthma by competitively binding CCND2 with miRNA-135a. Pulm Pharmacol Ther 77:102173. https://doi.org/10.1016/j.pupt.2022.102173
Liu SS, Bai YS, Feng L, Dong WW, Li Y, Xu LP, Ma NF (2016) Identification of CHD1L as an important regulator for spermatogonial stem cell survival and self-renewal. Stem Cells Int. https://doi.org/10.1155/2016/4069543
Tsuda M, Cho K, Ooka M, Shimizu N, Watanabe R, Yasui A, Nakazawa Y, Ogi T, Harada H, Agama K, Nakamura J, Asada R, Fujiike H, Sakuma T, Yamamoto T, Murai J, Hiraoka M, Koike K, Pommier Y, Takeda S, Hirota K (2017) ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PLoS ONE 12(11):e0188320. https://doi.org/10.1371/journal.pone.0188320
Wang L, Chen K, Chen Z (2021) Structural basis of ALC1/CHD1L autoinhibition and the mechanism of activation by the nucleosome. Nat Commun 12(1):4057. https://doi.org/10.1038/s41467-021-24320-4
Bekker-Jensen S, Rendtlew Danielsen J, Fugger K, Gromova I, Nerstedt A, Lukas C, Bartek J, Lukas J, Mailand N (2010) HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat Cell Biol 12(1):80–86. https://doi.org/10.1038/ncb2008. (sup pp 81-12)
Izawa N, Wu W, Sato K, Nishikawa H, Kato A, Boku N, Itoh F, Ohta T (2011) HERC2 interacts with claspin and regulates DNA origin firing and replication fork progression. Cancer Res 71(17):5621–5625. https://doi.org/10.1158/0008-5472.CAN-11-0385
Rodriguez-Berriguete G, Granata G, Puliyadi R, Tiwana G, Prevo R, Wilson RS, Yu S, Buffa F, Humphrey TC, McKenna WG, Higgins GS (2018) Nucleoporin 54 contributes to homologous recombination repair and post-replicative DNA integrity. Nucleic Acids Res 46(15):7731–7746. https://doi.org/10.1093/nar/gky569
Lancini C, van den Berk PC, Vissers JH, Gargiulo G, Song JY, Hulsman D, Serresi M, Tanger E, Blom M, Vens C, van Lohuizen M, Jacobs H, Citterio E (2014) Tight regulation of ubiquitin-mediated DNA damage response by USP3 preserves the functional integrity of hematopoietic stem cells. J Exp Med 211(9):1759–1777. https://doi.org/10.1084/jem.20131436
Nicassio F, Corrado N, Vissers JH, Areces LB, Bergink S, Marteijn JA, Geverts B, Houtsmuller AB, Vermeulen W, Di Fiore PP, Citterio E (2007) Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol 17(22):1972–1977. https://doi.org/10.1016/j.cub.2007.10.034
Takahashi A, Yousif A, Hong L, Chefetz I (2021) Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell. J Mol Med (Berl) 99(5):637–650. https://doi.org/10.1007/s00109-021-02055-5
Wang X, Zhang X, Dang Y, Li D, Lu G, Chan WY, Leung PCK, Zhao S, Qin Y, Chen ZJ (2020) Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res 48(8):4480–4491. https://doi.org/10.1093/nar/gkaa127
Yu T, Cazares O, Tang AD, Kim H-Y, Wald T, Verma A, Liu Q, Barcellos-Hoff MH, Floor SN, Jung H-S, Brooks AN, Klein OD (2022) SRSF1 governs progenitor-specific alternative splicing to maintain adult epithelial tissue homeostasis and renewal. Dev Cell 57(5):624-637.e624. https://doi.org/10.1016/j.devcel.2022.01.011
Acknowledgements
We thank Prof. Yuanchao Xue (Institute of Biophysics, Chinese Academy of Sciences, Beijing) for sharing Srsf1Fl/Fl mice, Prof. Chao Wang (China Agricultural University, Beijing) and Shuyang Yu for thoughtful discussions and suggestions, and all the members of Prof. Hua Zhang and Chao Wang laboratory for helpful discussions and comments. We thank Novogene for their assistance with the RNA-seq experiments.
Funding
This work was supported by the National Key Research & Developmental Program of China [2021YFF1000603; 2018YFC1003701]. Funding for open access charge: China Agricultural University.
Author information
Authors and Affiliations
Contributions
XY, CW, HZ, and JL conceived and designed the entire project. XY, CW, and WY performed the experiments. XY, CW, WY, LS, ZL, XX, ST, and LY contributed to breeding mice. XY, CW, JL, and WY analyzed the data. XY, CW, and JL wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest statement. None declared.
Ethics approval and consent to participate
This study was approved by China Agricultural University laboratory animal welfare and animal experimental ethical inspection (Issue No. AW11402202-3–6).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, X., Wang, C., Yu, W. et al. SRSF1 is essential for primary follicle development by regulating granulosa cell survival via mRNA alternative splicing. Cell. Mol. Life Sci. 80, 343 (2023). https://doi.org/10.1007/s00018-023-04979-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04979-2