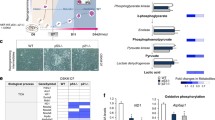
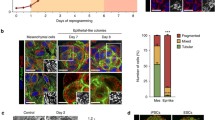
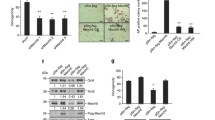
Abstract
Mitochondria are versatile organelles that continuously change their morphology via fission and fusion. However, the detailed functions of mitochondrial dynamics-related genes in pluripotent stem cells remain largely unclear. Here, we aimed to determine the effects on energy metabolism and differentiation ability of mouse embryonic stem cells (ESCs) following deletion of the mitochondrial fission-related gene Dnml1. Resultant Dnm1l−/− ESCs maintained major pluripotency characteristics. However, Dnm1l−/− ESCs showed several phenotypic changes, including the inhibition of differentiation ability (dissolution of pluripotency). Notably, Dnm1l−/− ESCs maintained the expression of the pluripotency marker Oct4 and undifferentiated colony types upon differentiation induction. RNA sequencing analysis revealed that the most frequently differentially expressed genes were enriched in the glutathione metabolic pathway. Our data suggested that differentiation inhibition of Dnm1l−/− ESCs was primarily due to metabolic shift from glycolysis to OXPHOS, G2/M phase retardation, and high level of Nanog and 2-cell-specific gene expression.






Similar content being viewed by others
Availability of data and material
Data will be available on reasonable request.
References
Baek SH, Park SJ, Jeong JI et al (2017) Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer’s disease model. J Neurosci 37:5099–5110
Boroviak T, Loos R, Bertone P et al (2014) The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol 16:513–525
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45:466–472
Chan DC (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46:265–287
Chang DT, Honick AS, Reynolds IJ (2006) Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci 26:7035–7045
Choi HW, Joo JY, Hong YJ et al (2016) Distinct enhancer activity of Oct4 in naive and primed mouse pluripotency. Stem Cell Rep 7:911–926
Choi J, Seo BJ, La H et al (2020) Comparative analysis of the mitochondrial morphology, energy metabolism, and gene expression signatures in three types of blastocyst-derived stem cells. Redox Biol 30:101437
Chung S, Arrell DK, Faustino RS et al (2010) Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol 48:725–734
Coronado D, Godet M, Bourillot P-Y et al (2013) A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res 10:118–131
Dan J, Rousseau P, Hardikar S et al (2017) Zscan4 inhibits maintenance DNA methylation to facilitate telomere elongation in mouse embryonic stem cells. Cell Rep 20:1936–1949
Devoto VMP, Falzone TL (2017) Mitochondrial dynamics in Parkinson’s disease: a role for α-synuclein? Dis Model Mech 10:1075–1087
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Facucho-Oliveira J, John JS (2009) The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev Rep 5:140–158
Facucho-Oliveira JM, Alderson J, Spikings EC et al (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120:4025–4034
Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13:75–88
Folmes CD, Dzeja PP, Nelson TJ et al (2012) Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11:596–606
Folmes CD, Nelson TJ, Martinez-Fernandez A et al (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14:264–271
Giacomello M, Pyakurel A, Glytsou C et al (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21:204–224
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Heurtier V, Owens N, Gonzalez I et al (2019) The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat Commun 10:1–15
Ishihara N, Nomura M, Jofuku A et al (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11:958–966
Kansanen E, Kuosmanen SM, Leinonen H et al (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1:45–49
Kasai S, Shimizu S, Tatara Y et al (2020) Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 10:320
Kauffman ME, Kauffman MK, Traore K et al (2016) MitoSOX-based flow cytometry for detecting mitochondrial ROS. React Oxyg Species (Apex, NC) 2:361
Ko E, Seo HW, Jung G (2018) Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology 67:1378–1391
Kondoh H, Lleonart ME, Nakashima Y et al (2007) A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 9:293–299
Kowaltowski AJ, De Souza-Pinto NC, Castilho RF et al (2009) Mitochondria and reactive oxygen species. Free Radical Biol Med 47:333–343
Lapasset L, Milhavet O, Prieur A et al (2011) Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 25:2248–2253
Li Z, Okamoto K-I, Hayashi Y et al (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887
Lonergan T, Bavister B, Brenner C (2007) Mitochondria in stem cells. Mitochondrion 7:289–296
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Mandal S, Freije WA, Guptan P et al (2010) Metabolic control of G1–S transition: cyclin E degradation by p53-induced activation of the ubiquitin–proteasome system. J Cell Biol 188:473–479
Martello G, Smith A (2014) The nature of embryonic stem cells. Annu Rev Cell Dev Biol 30:647–675
Mitra K, Wunder C, Roysam B et al (2009) A hyperfused mitochondrial state achieved at G1–S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci 106:11960–11965
Morales PE, Arias-Durán C, Ãvalos-Guajardo Y et al (2020) Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med 71:100822
Mullarky E, Cantley LC (2015) Diverting glycolysis to combat oxidative stress. Innov Med. 3–23
Murnane JP (2012) Telomere dysfunction and chromosome instability. Mutat Res Fundam Mol Mech Mutagen 730:28–36
Nakai-Futatsugi Y, Niwa H (2016) Zscan4 is activated after telomere shortening in mouse embryonic stem cells. Stem Cell Rep 6:483–495
Namani A, Rahaman MM, Chen M et al (2018) Gene-expression signature regulated by the KEAP1-NRF2-CUL3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer 18:46
Oliver D, Reddy PH (2019) Dynamics of Dynamin-related protein 1 in Alzheimer’s disease and other neurodegenerative diseases. Cells 8:961
Otera H, Ishihara N, Mihara K (2013) New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta (BBA) Mol Cell Res 1833:1256–1268
Pauklin S, Vallier L (2013) The cell-cycle state of stem cells determines cell fate propensity. Cell 155:135–147
Qian W, Choi S, Gibson GA et al (2012) Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci 125:5745–5757
Ran FA, Hsu PD, Wright J et al (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281
Reddy PH (2009) Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol 218:286–292
Rodriguez-Terrones D, Gaume X, Ishiuchi T et al (2018) A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nat Genet 50:106–119
Salazar-Roa M, Malumbres M (2017) Fueling the cell division cycle. Trends Cell Biol 27:69–81
Seo BJ, Choi J, La H et al (2020) Role of mitochondrial fission-related genes in mitochondrial morphology and energy metabolism in mouse embryonic stem cells. Redox Biol 36:101599
Seo BJ, Yoon SH, Do JT (2018) Mitochondrial dynamics in stem cells and differentiation. Int J Mol Sci 19:3893
Song M, Mihara K, Chen Y et al (2015) Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21:273–286
Taguchi N, Ishihara N, Jofuku A et al (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282:11521–11529
Tataranni T, Agriesti F, Pacelli C et al (2019) Dichloroacetate affects mitochondrial function and stemness-associated properties in pancreatic cancer cell lines. Cells 8:478
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29:1727–1745
Torre D, Lachmann A, Maayan A (2018) BioJupies: automated generation of interactive notebooks for RNA-Seq data analysis in the cloud. Cell Syst 7:556-561 e553
Vardhana SA, Arnold PK, Rosen BP et al (2019) Glutamine independence is a selectable feature of pluripotent stem cells. Nat Metab 1:676–687
Von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Von Zglinicki T, Saretzki G, Döcke W et al (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220:186–193
Wai T, Langer T (2016) Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab 27:105–117
Wakabayashi J, Zhang Z, Wakabayashi N et al (2009) The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186:805–816
Wang W, Yin J, Ma X et al (2017) Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum Mol Genet 26:4118–4131
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884
White J, Dalton S (2005) Cell cycle control of embryonic stem cells. Stem Cell Rev 1:131–138
Xu X, Duan S, Yi F et al (2013) Mitochondrial regulation in pluripotent stem cells. Cell Metab 18:325–332
Yang J, Guo R, Wang H et al (2016) Tet enzymes regulate telomere maintenance and chromosomal stability of mouse ESCs. Cell Rep 15:1809–1821
Yeh CD, Richardson CD, Corn JE (2019) Advances in genome editing through control of DNA repair pathways. Nat Cell Biol 21:1468–1478
Ying Q-L, Smith AG (2003) Defined conditions for neural commitment and differentiation. Methods Enzymol 365:327–341
Zalzman M, Falco G, Sharova LV et al (2010) Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464:858–863
Zhang J, Nuebel E, Daley GQ et al (2012) Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11:589–595
Zhang Q, Dan J, Wang H et al (2016) Tcstv1 and Tcstv3 elongate telomeres of mouse ES cells. Sci Rep 6:19852
Zhou W, Choi M, Margineantu D et al (2012) HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31:2103–2116
Zunino R, Braschi E, Xu L et al (2009) Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem 284:17783–17795
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) of the Republic of Korea (Grant No. RS-2023-00208330) and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET), funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant No. 322006-05-02-CG000).
Author information
Authors and Affiliations
Contributions
BJS and JTD: wrote the main manuscript and designed the study. BJS, SBN, and JC: performed experiments and analyzed data. BJS, BA, CP, KH, and JTD: performed data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
Experiments were performed following the approved guidelines and all experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, B.J., Na, S.B., Choi, J. et al. Metabolic and cell cycle shift induced by the deletion of Dnm1l attenuates the dissolution of pluripotency in mouse embryonic stem cells. Cell. Mol. Life Sci. 80, 302 (2023). https://doi.org/10.1007/s00018-023-04962-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04962-x