Abstract
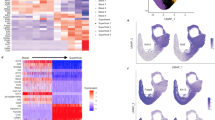
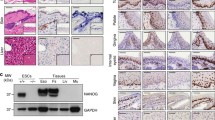
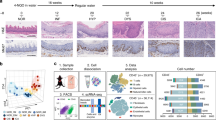
Self-renewing, damage–repair and differentiation of mammalian stratified squamous epithelia are subject to tissue homeostasis, but the regulation mechanisms remain elusive. Here, we investigate the esophageal squamous epithelial tissue homeostasis in vitro and in vivo. We establish a rat esophageal organoid (rEO) in vitro system and show that the landscapes of rEO formation, development and maturation trajectories can mimic those of rat esophageal epithelia in vivo. Single-cell RNA sequencing (scRNA-seq), snapshot immunostaining and functional analyses of stratified “matured” rEOs define that the epithelial pluripotent stem cell determinants, p63 and Sox2, play crucial but distinctive roles for regulating mammalian esophageal tissue homeostasis. We identify two cell populations, p63+Sox2+ and p63−Sox2+, of which the p63+Sox2+ population presented at the basal layer is the cells of origin required for esophageal epithelial stemness maintenance and proliferation, whereas the p63−Sox2+ population presented at the suprabasal layers is the cells of origin having a dual role for esophageal epithelial differentiation (differentiation-prone fate) and rapid tissue damage–repair responses (proliferation-prone fate). Given the fact that p63 and Sox2 are developmental lineage oncogenes and commonly overexpressed in ESCC tissues, p63−Sox2+ population could not be detected in organoids formed by esophageal squamous cell carcinoma (ESCC) cell lines. Taken together, these findings reveal that the tissue homeostasis is maintained distinctively by p63 and/or Sox2-dependent cell lineage populations required for the tissue renewing, damage–repair and protection of carcinogenesis in mammalian esophagi.







Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
Abbreviations
- rEO:
-
Rat esophageal organoid
- scRNA-seq:
-
Single-cell RNA sequencing
- ESCC:
-
Esophageal squamous cell carcinoma
- RES:
-
Respiratory–esophageal separation
- SRY:
-
Sex-determining region Y
- HDs:
-
Hemidesmosomes
- mEOs:
-
Mammalian esophageal organoids
- PSCs:
-
Pluripotent stem cells
- EoE:
-
Eosinophilic esophagitis
- SOE:
-
Overexpression of Sox2
- hTERT:
-
Human telomere reverse transcriptase
- RNE-D3:
-
Immortalized rat normal esophageal epithelial D3 cell line
- RESCs:
-
Rat esophageal squamous carcinoma cell lines
- FBS:
-
Fetal bovine serum
- OFR:
-
Organoid formation rate
- PCs:
-
Primary rat esophageal squamous keratinocyte cells
References
Kuwahara A, Lewis AE, Coombes C, Leung FS, Percharde M, Bush JO (2020) Delineating the early transcriptional specification of the mammalian trachea and esophagus. Elife. https://doi.org/10.7554/eLife.55526
Zhang Y, Jiang M, Kim E, Lin S, Liu K, Lan X et al (2017) Development and stem cells of the esophagus. Semin Cell Dev Biol. 66:25–35. https://doi.org/10.1016/j.semcdb.2016.12.008
Getachew D, Kaneda R, Saeki Y, Matsumoto A, Otani H (2021) Morphologic changes in the cytoskeleton and adhesion apparatus during the conversion from pseudostratified single columnar to stratified squamous epithelium in the developing mouse esophagus. Congenit Anom (Kyoto). 61(1):14–24. https://doi.org/10.1111/cga.12389
Whelan KA, Muir AB, Nakagawa H (2018) Esophageal 3D culture systems as modeling tools in esophageal epithelial pathobiology and personalized medicine. Cell Mol Gastroenterol Hepatol. 5(4):461–78. https://doi.org/10.1016/j.jcmgh.2018.01.011
Que J (2015) The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus. Wiley Interdiscip Rev Dev Biol. 4(4):419–30. https://doi.org/10.1002/wdev.179
Kim E, Jiang M, Huang H, Zhang Y, Tjota N, Gao X et al (2019) Isl1 regulation of Nkx2.1 in the early foregut epithelium is required for trachea-esophageal separation and lung lobation. Dev Cell. 51(6):675–83. https://doi.org/10.1016/j.devcel.2019.11.002
Teramoto M, Sugawara R, Minegishi K, Uchikawa M, Takemoto T, Kuroiwa A et al (2020) The absence of SOX2 in the anterior foregut alters the esophagus into trachea and bronchi in both epithelial and mesenchymal components. Biol Open. https://doi.org/10.1242/bio.048728
Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE et al (2007) Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 134(13):2521–31. https://doi.org/10.1242/dev.003855
Trisno SL, Philo KED, McCracken KW, Cata EM, Ruiz-Torres S, Rankin SA et al (2018) Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell. 23(4):501-515 e7. https://doi.org/10.1016/j.stem.2018.08.008
Morrisey EE, Rustgi AK (2018) The lung and esophagus: developmental and regenerative overlap. Trends Cell Biol. 28(9):738–48. https://doi.org/10.1016/j.tcb.2018.04.007
Jiang YY, Jiang Y, Li CQ, Zhang Y, Dakle P, Kaur H et al (2020) TP63, SOX2, and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology. 159(4):1311-1327 e19. https://doi.org/10.1053/j.gastro.2020.06.050
Sastre-Perona A, Hoang-Phou S, Leitner MC, Okuniewska M, Meehan S, Schober M (2019) De Novo PITX1 expression controls bi-stable transcriptional circuits to govern self-renewal and differentiation in squamous cell carcinoma. Cell Stem Cell. 24(3):390-404.e8. https://doi.org/10.1016/j.stem.2019.01.003
Porter L, McCaughan F (2020) SOX2 and squamous cancers. Semin Cancer Biol. 67(Pt 1):154–67. https://doi.org/10.1016/j.semcancer.2020.05.007
Rosekrans SL, Baan B, Muncan V, van den Brink GR (2015) Esophageal development and epithelial homeostasis. Am J Physiol Gastrointest Liver Physiol. 309(4):G216-28. https://doi.org/10.1152/ajpgi.00088.2015
Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH et al (2004) Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 287(1):171–181. https://doi.org/10.1152/ajpcell.00226.2003
Yu WY, Slack JM, Tosh D (2005) Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev Biol. 284(1):157–70. https://doi.org/10.1016/j.ydbio.2005.04.042
Kajiwara C, Fumoto K, Kimura H, Nojima S, Asano K, Odagiri K et al (2018) p63-dependent dickkopf3 expression promotes esophageal cancer cell proliferation via CKAP4. Cancer Res. 78(21):6107–20. https://doi.org/10.1158/0008-5472.CAN-18-1749
Zhang Y, Yang Y, Jiang M, Huang SX, Zhang W, Al Alam D et al (2018) 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell. 23(4):516–29. https://doi.org/10.1016/j.stem.2018.08.009
Piedrafita G, Kostiou V, Wabik A, Colom B, Fernandez-Antoran D, Herms A et al (2020) A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nat Commun. 11(1):1429. https://doi.org/10.1038/s41467-020-15258-0
Kijima T, Nakagawa H, Shimonosono M, Chandramouleeswaran PM, Hara T, Sahu V et al (2019) Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 7(1):73–91. https://doi.org/10.1016/j.jcmgh.2018.09.003
Yao J, Cui Q, Fan W, Ma Y, Chen Y, Liu T et al (2020) Single-cell transcriptomic analysis in a mouse model deciphers cell transition states in the multistep development of esophageal cancer. Nat Commun. 11(1):3715. https://doi.org/10.1038/s41467-020-17492-y
Wu Z, Zhou J, Zhang X, Zhang Z, Xie Y, Liu JB et al (2021) Reprogramming of the esophageal squamous carcinoma epigenome by SOX2 promotes ADAR1 dependence. Nat Genet. 53(6):881–94. https://doi.org/10.1038/s41588-021-00859-2
Colom B, Alcolea MP, Piedrafita G, Hall MWJ, Wabik A, Dentro SC et al (2020) Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat Genet. 52(6):604–14. https://doi.org/10.1038/s41588-020-0624-3
Yang Y, Deng G, Qiao L, Yuan H, Yu X, Xu L et al (2022) Identification and characterization of stem cells in mammalian esophageal stratified squamous epithelia. J Mol Cell Biol. https://doi.org/10.1093/jmcb/mjac038
Busslinger GA, Weusten BLA, Bogte A, Begthel H, Brosens LAA, Clevers H (2021) Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 34(10):108819. https://doi.org/10.1016/j.celrep.2021.108819
Kabir MF, Karami AL, Cruz-Acuña R, Klochkova A, Saxena R, Mu A et al (2022) Single cell transcriptomic analysis reveals cellular diversity of murine esophageal epithelium. Nat Commun. 13(1):2167. https://doi.org/10.1038/s41467-022-29747-x
Liu N, Matsumura H, Kato T, Ichinose S, Takada A, Namiki T et al (2019) Stem cell competition orchestrates skin homeostasis and ageing. Nature 568(7752):344–350. https://doi.org/10.1038/s41586-019-1085-7
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Zheng B, Ko KP, Fang X, Wang X, Zhang J, Jun S et al (2021) A new murine esophageal organoid culture method and organoid-based model of esophageal squamous cell neoplasia. iScience. 24(12):103440. https://doi.org/10.1016/j.isci.2021.103440
Shacham-Silverberg V, Wells JM (2020) Generation of esophageal organoids and organotypic raft cultures from human pluripotent stem cells. Methods Cell Biol. 159:1–22. https://doi.org/10.1016/bs.mcb.2020.04.009
Rustgi AK (2018) 3D human esophageal epithelium steps out from hPSCs. Cell Stem Cell. 23(4):460–2. https://doi.org/10.1016/j.stem.2018.09.010
Nakagawa H, Kasagi Y, Karakasheva TA, Hara T, Aaron B, Shimonosono M et al (2020) Modeling epithelial homeostasis and reactive epithelial changes in human and murine three-dimensional esophageal organoids. Curr Protoc Stem Cell Biol. 52(1):e106. https://doi.org/10.1002/cpsc.106
Raad S, David A, Que J, Faure C (2020) Genetic mouse models and induced pluripotent stem cells for studying tracheal-esophageal separation and esophageal development. Stem Cells Dev. 29(15):953–66. https://doi.org/10.1089/scd.2020.0075
Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M et al (2018) The esophageal organoid system reveals functional interplay between notch and cytokines in reactive epithelial changes. Cell Mol Gastroenterol Hepatol. 5(3):333–52. https://doi.org/10.1016/j.jcmgh.2017.12.013
Hara T, Kasagi Y, Wang J, Sasaki M, Aaron B, Karami A et al (2022) CD73(+) epithelial progenitor cells that contribute to homeostasis and renewal are depleted in eosinophilic esophagitis. Cell Mol Gastroenterol Hepatol. 13(5):1449–67. https://doi.org/10.1016/j.jcmgh.2022.01.018
Karakasheva TA, Kijima T, Shimonosono M, Maekawa H, Sahu V, Gabre JT et al (2020) Generation and characterization of patient-derived head and neck, oral, and esophageal cancer organoids. Curr Protoc Stem Cell Biol. 53(1):e109. https://doi.org/10.1002/cpsc.109
Doyle AD, Masuda MY, Pyon GC, Luo H, Putikova A, LeSuer WE et al (2023) Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy. 78(1):192–201. https://doi.org/10.1111/all.15457
Sachdeva UM, Shimonosono M, Flashner S, Cruz-Acuna R, Gabre JT, Nakagawa H (2021) Understanding the cellular origin and progression of esophageal cancer using esophageal organoids. Cancer Lett. 509:39–52. https://doi.org/10.1016/j.canlet.2021.03.031
Zhang Y, Bailey D, Yang P, Kim E, Que J (2021) The development and stem cells of the esophagus. Development. https://doi.org/10.1242/dev.193839
O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT et al (2018) Pathophysiology of eosinophilic esophagitis. Gastroenterology. 154(2):333–45. https://doi.org/10.1053/j.gastro.2017.06.065
Gen Y, Yasui K, Zen Y, Zen K, Dohi O, Endo M et al (2010) SOX2 identified as a target gene for the amplification at 3q26 that is frequently detected in esophageal squamous cell carcinoma. Cancer Genet Cytogenet. 202(2):82–93. https://doi.org/10.1016/j.cancergencyto.2010.01.023
Monceviciūte-Eringiene E (2005) Neoplastic growth: the consequence of evolutionary malignant resistance to chronic damage for survival of cells (review of a new theory of the origin of cancer). Med Hypotheses. 65(3):595–604. https://doi.org/10.1016/j.mehy.2005.02.033
Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmoulière A (2012) The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 5(Suppl 1):S5. https://doi.org/10.1186/1755-1536-5-s1-s5
Zou Z, Zheng W, Fan H, Deng G, Lu SH, Jiang W et al (2021) Aspirin enhances the therapeutic efficacy of cisplatin in oesophageal squamous cell carcinoma by inhibition of putative cancer stem cells. Br J Cancer. 125(6):826–38. https://doi.org/10.1038/s41416-021-01499-3
Fan H, Yu X, Zou Z, Zheng W, Deng X, Guo L et al (2019) Metformin suppresses the esophageal carcinogenesis in rats treated with NMBzA through inhibiting AMPK/mTOR signaling pathway. Carcinogenesis. 40(5):669–79. https://doi.org/10.1093/carcin/bgy160
Saladi SV, Ross K, Karaayvaz M, Tata PR, Mou H, Rajagopal J et al (2017) ACTL6A is co-amplified with p63 in squamous cell carcinoma to drive YAP activation, regenerative proliferation, and poor prognosis. Cancer Cell. 31(1):35–49. https://doi.org/10.1016/j.ccell.2016.12.001
Liu Y, Yin N, Wang X, Khoor A, Sambandam V, Ghosh AB et al (2020) Chromosome 3q26 gain is an early event driving coordinated overexpression of the PRKCI, SOX2, and ECT2 oncogenes in lung squamous cell carcinoma. Cell Rep. 30(3):771–82 e6. https://doi.org/10.1016/j.celrep.2019.12.071
Bass AJ, Wang TC (2013) An inflammatory situation: SOX2 and STAT3 cooperate in squamous cell carcinoma initiation. Cell Stem Cell. 12(3):266–8. https://doi.org/10.1016/j.stem.2013.02.004
Hsieh MH, Choe JH, Gadhvi J, Kim YJ, Arguez MA, Palmer M et al (2019) p63 and SOX2 dictate glucose reliance and metabolic vulnerabilities in squamous cell carcinomas. Cell Rep. 28(7):1860–78.e9. https://doi.org/10.1016/j.celrep.2019.07.027
Liu K, Jiang M, Lu Y, Chen H, Sun J, Wu S et al (2013) Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 12(3):304–15. https://doi.org/10.1016/j.stem.2013.01.007
Li L, Wang Y, Torkelson JL, Shankar G, Pattison JM, Zhen HH et al (2019) TFAP2C- and p63-dependent networks sequentially rearrange chromatin landscapes to drive human epidermal lineage commitment. Cell Stem Cell. 24(2):271–84.e8. https://doi.org/10.1016/j.stem.2018.12.012
Smirnov A, Lena AM, Cappello A, Panatta E, Anemona L, Bischetti S et al (2019) ZNF185 is a p63 target gene critical for epidermal differentiation and squamous cell carcinoma development. Oncogene. 38(10):1625–38. https://doi.org/10.1038/s41388-018-0509-4
Dunn JLM, Caldwell JM, Ballaban A, Ben-Baruch Morgenstern N, Rochman M, Rothenberg ME (2021) Bidirectional crosstalk between eosinophils and esophageal epithelial cells regulates inflammatory and remodeling processes. Mucosal Immunol. 14(5):1133–43. https://doi.org/10.1038/s41385-021-00400-y
DeWard AD, Cramer J, Lagasse E (2014) Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9(2):701–11. https://doi.org/10.1016/j.celrep.2014.09.027
Li L, Wang Y, Torkelson JL, Shankar G, Pattison JM, Zhen HH et al (2019) TFAP2C- and p63-dependent networks sequentially rearrange chromatin landscapes to drive human epidermal lineage commitment. Cell Stem Cell. 24(2):271–84 e8. https://doi.org/10.1016/j.stem.2018.12.012
Senoo M, Pinto F, Crum CP, McKeon F (2007) p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 129(3):523–36. https://doi.org/10.1016/j.cell.2007.02.045
Ameis D, Liu F, Kirby E, Patel D, Keijzer R (2021) The RNA-binding protein Quaking regulates multiciliated and basal cell abundance in the developing lung. Am J Physiol Lung Cell Mol Physiol 320(4):L557–L567. https://doi.org/10.1152/ajplung.00481.2019
Giandomenico SL, Sutcliffe M, Lancaster MA (2021) Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat Protoc. 16(2):579–602. https://doi.org/10.1038/s41596-020-00433-w
Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T (2015) Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci USA. 112(10):3002–7. https://doi.org/10.1073/pnas.1420024112
de Medeiros G, Ortiz R, Strnad P, Boni A, Moos F, Repina N et al (2022) Multiscale light-sheet organoid imaging framework. Nat Commun. 13(1):4864. https://doi.org/10.1038/s41467-022-32465-z
Acknowledgements
During this study (in 2019), Prof. Shih-Hsin Lu passed away. We all miss him.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (Gran number 81972572 to Wei Jiang) and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (Grant number 2021-I2M-1-014 to Xiying Yu).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xiaohong Yu and HY. The first draft of the manuscript was written by Xiying Yu and WJ. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The remaining authors declare no competing interests. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All experiments involving animals were complied with the standards approved by ethical committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, X., Yuan, H., Yang, Y. et al. Mammalian esophageal stratified tissue homeostasis is maintained distinctively by the epithelial pluripotent p63+Sox2+ and p63−Sox2+ cell populations. Cell. Mol. Life Sci. 80, 305 (2023). https://doi.org/10.1007/s00018-023-04952-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04952-z