Abstract
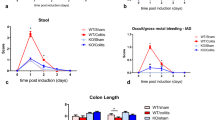
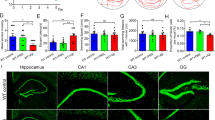
Alzheimer's disease (AD) is traditionally considered as a brain disorder featured by amyloid-β (Aβ) deposition. The current study on whether pathological changes of AD extend to the enteric nervous system (ENS) is still in its infancy. In this study, we found enteric Aβ deposition, intestinal dysfunction, and colonic inflammation in the young APP/PS1 mice. Moreover, these mice exhibited cholinergic and nitrergic signaling pathways damages and enteric neuronal loss. Our data show that Aβ42 treatment remarkably affected the gene expression of cultured myenteric neurons and the spontaneous contraction of intestinal smooth muscles. The intra-colon administration of Aβ42 induced ENS dysfunction, brain gliosis, and β-amyloidosis-like changes in the wild-type mice. Our results suggest that ENS mirrors the neuropathology observed in AD brains, and intestinal pathological changes may represent the prodromal events, which contribute to brain pathology in AD. In summary, our findings provide new opportunities for AD early diagnosis and prevention.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K (2021) Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y) 7:e12179
Wang J, Gu BJ, Masters CL, Wang YJ (2017) A systemic view of Alzheimer disease—insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13:612–623
Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM et al (2009) Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement 5:18–29
Sun HL, Chen SH, Yu ZY, Cheng Y, Tian DY, Fan DY, He CY, Wang J, Sun PY, Chen Y et al (2021) Blood cell-produced amyloid-β induces cerebral Alzheimer-type pathologies and behavioral deficits. Mol Psychiatry 26:5568–5577
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155
Niesler B, Kuerten S, Demir IE, Schäfer KH (2021) Disorders of the enteric nervous system—a holistic view. Nat Rev Gastroenterol Hepatol 18:393–410
Sohrabi M, Pecoraro HL, Combs CK (2021) Gut Inflammation induced by dextran sulfate sodium exacerbates amyloid-β plaque deposition in the AppNL-G-F mouse model of Alzheimer’s disease. J Alzheimers Dis 79:1235–1255
Chen C, Ahn EH, Kang SS, Liu X, Alam A, Ye K (2020) Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci Adv 6:eaba0466
Wu SC, Cao ZS, Chang KM, Juang JL (2017) Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat Commun 8:24
Kim MS, Kim Y, Choi H, Kim W, Park S, Lee D, Kim DK, Kim HJ, Choi H, Hyun DW et al (2020) Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69:283–294
Chalazonitis A, Rao M (2018) Enteric nervous system manifestations of neurodegenerative disease. Brain Res 1693:207–213
Schneider S, Wright CM, Heuckeroth RO (2019) Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol 81:235–259
Wang L, Fleming SM, Chesselet M-F, Taché Y (2008) Abnormal colonic motility in mice overexpressing human wild-type α-synuclein. NeuroReport 19:873
Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, Taché Y (2012) Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil 24:e425–e436
Kim S, Kwon S-H, Kam T-I, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103:627-641.e627
Wang X, Hu X, Yang Y, Takata T, Sakurai T (2016) Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res 1643:1–9
Puig KL, Combs CK (2013) Expression and function of APP and its metabolites outside the central nervous system. Exp Gerontol 48:608–611
Puig KL, Lutz BM, Urquhart SA, Rebel AA, Zhou X, Manocha GD, Sens M, Tuteja AK, Foster NL, Combs CK (2015) Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system. J Alzheimers Dis 44:1263–1278
Puig KL, Brose SA, Zhou X, Sens MA, Combs GF, Jensen MD, Golovko MY, Combs CK (2017) Amyloid precursor protein modulates macrophage phenotype and diet-dependent weight gain. Sci Rep 7:43725
Puig KL, Manocha GD, Combs CK (2015) Amyloid precursor protein mediated changes in intestinal epithelial phenotype in vitro. PLoS One 10:e0119534
Cabal A, Alonso-Cortina V, Gonzalez-Vazquez LO, Naves FJ, Del Valle ME, Vega JA (1995) beta-Amyloid precursor protein (beta APP) in human gut with special reference to the enteric nervous system. Brain Res Bull 38:417–423
Vojtechova I, Machacek T, Kristofikova Z, Stuchlik A, Petrasek T (2022) Infectious origin of Alzheimer’s disease: amyloid beta as a component of brain antimicrobial immunity. PLoS Pathog 18:e1010929
Li B, He Y, Ma J, Huang P, Du J, Cao L, Wang Y, Xiao Q, Tang H, Chen S (2019) Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement 15:1357–1366
Zhao L, Lin S, Bales KR, Gelfanova V, Koger D, Delong C, Hale J, Liu F, Hunter JM, Paul SM (2009) Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J Neurosci 29:3603–3612
Welikovitch LA, Do Carmo S, Maglóczky Z, Malcolm JC, Lőke J, Klein WL, Freund T, Cuello AC (2020) Early intraneuronal amyloid triggers neuron-derived inflammatory signaling in APP transgenic rats and human brain. Proc Natl Acad Sci USA 117:6844–6854
Bayer TA, Wirths O (2010) Intracellular accumulation of amyloid-Beta—a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front Aging Neurosci 2:8
Khodabakhsh P, Bazrgar M, Dargahi L, Mohagheghi F, Asgari Taei A, Parvardeh S, Ahmadiani A (2021) Does Alzheimer’s disease stem in the gastrointestinal system? Life Sci 287:120088
Sanders KM, Koh SD, Ro S, Ward SM (2012) Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9:633–645
Sanders KM, Ward SM (2019) Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br J Pharmacol 176:212–227
El-Yazbi AF, Cho WJ, Cena J, Schulz R, Daniel EE (2008) Smooth muscle NOS, colocalized with caveolin-1, modulates contraction in mouse small intestine. J Cell Mol Med 12:1404–1415
Beck K, Friebe A, Voussen B (2018) Nitrergic signaling via interstitial cells of Cajal and smooth muscle cells influences circular smooth muscle contractility in murine colon. Neurogastroenterol Motil 30:e13300
Bódi N, Szalai Z, Bagyánszki M (2019) Nitrergic enteric neurons in health and disease-focus on animal models. Int J Mol Sci 20:2003
Iwasaki M, Akiba Y, Kaunitz JD (2019) Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system. F1000Res. https://doi.org/10.12688/f1000research.18039.1
Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S (2012) Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143:1006-1016.e4
Caputi V, Marsilio I, Cerantola S, Roozfarakh M, Lante I, Galuppini F, Rugge M, Napoli E, Giulivi C, Orso G et al (2017) Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front Pharmacol 8:350
Ye L, Li G, Goebel A, Raju AV, Kong F, Lv Y, Li K, Zhu Y, Raja S, He P et al (2020) Caspase-11-mediated enteric neuronal pyroptosis underlies Western diet-induced colonic dysmotility. J Clin Investig 130:3621–3636
Heanue TA, Pachnis V (2011) Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells 29:128–140
Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C et al (2017) Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA 114:E3709–E3718
Kesavardhana S, Malireddi RKS, Kanneganti TD (2020) Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol 38:567–595
Han XJ, Hu YY, Yang ZJ, Jiang LP, Shi SL, Li YR, Guo MY, Wu HL, Wan YY (2017) Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol Med Rep 16:4521–4528
Zhan X, Stamova B, Sharp FR (2018) Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: a review. Front Aging Neurosci 10:42
Brown GC (2010) Nitric oxide and neuronal death. Nitric Oxide 23:153–165
Ghasemi M, Mayasi Y, Hannoun A, Eslami SM, Carandang R (2018) Nitric oxide and mitochondrial function in neurological diseases. Neuroscience 376:48–71
Delgado M, Varela N, Gonzalez-Rey E (2008) Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia 56:1091–1103
Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M (2010) Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330:980–982
Zhang XM, Xiong K, Cai Y, Cai H, Luo XG, Feng JC, Clough RW, Patrylo PR, Struble RG, Yan XX (2010) Functional deprivation promotes amyloid plaque pathogenesis in Tg2576 mouse olfactory bulb and piriform cortex. Eur J Neurosci 31:710–721
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 14:750–756
Saiz-Sanchez D, Ubeda-Bañon I, De la Rosa-Prieto C, Martinez-Marcos A (2012) Differential expression of interneuron populations and correlation with amyloid-β deposition in the olfactory cortex of an AβPP/PS1 transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 31:113–129
Rao M, Gershon MD (2016) The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 13:517–528
Brooks AJ, Rowse G, Ryder A, Peach EJ, Corfe BM, Lobo AJ (2016) Systematic review: psychological morbidity in young people with inflammatory bowel disease - risk factors and impacts. Aliment Pharmacol Ther 44:3–15
Custodia A, Ouro A, Romaus-Sanjurjo D, Pías-Peleteiro JM, de Vries HE, Castillo J, Sobrino T (2021) Endothelial progenitor cells and vascular alterations in Alzheimer’s disease. Front Aging Neurosci 13:811210
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, Lü Y, Cai M, Zhu C, Tan YL et al (2018) Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63:1337–1346
Feng J, Dong L, Zhang J, Han X, Tang S, Song L, Cong L, Wang X, Wang Y, Du Y (2018) Unique expression pattern of KIBRA in the enteric nervous system of APP/PS1 mice. Neurosci Lett 675:41–47
Manocha GD, Floden AM, Miller NM, Smith AJ, Nagamoto-Combs K, Saito T, Saido TC, Combs CK (2019) Temporal progression of Alzheimer’s disease in brains and intestines of transgenic mice. Neurobiol Aging 81:166–176
Li JW, Zong Y, Cao XP, Tan L, Tan L (2018) Microglial priming in Alzheimer’s disease. Ann Transl Med 6:176
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM et al (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156:1045–1059
Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD (2012) NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488:389–393
Stoye NM, Dos Santos Guilherme M, Endres K (2020) Alzheimer’s disease in the gut-Major changes in the gut of 5xFAD model mice with ApoA1 as potential key player. FASEB J 34:11883–11899
Sohrabi M, Sahu B, Kaur H, Hasler WA, Prakash A, Combs CK (2022) Gastrointestinal changes and Alzheimer’s disease. Curr Alzheimer Res 19:335–350
Furness JB, Stebbing MJ (2018) The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil. https://doi.org/10.1111/nmo.13234
Chen C, Zhou Y, Wang H, Alam A, Kang SS, Ahn EH, Liu X, Jia J, Ye K (2021) Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer’s disease. EMBO J 40:e106320
Pellegrini C, Daniele S, Antonioli L, Benvenuti L, D’Antongiovanni V, Piccarducci R, Pietrobono D, Citi V, Piragine E, Flori L et al (2020) Prodromal intestinal events in Alzheimer’s disease (AD): colonic dysmotility and inflammation are associated with enteric AD-related protein deposition. Int J Mol Sci 21:3523
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ et al (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933
Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, Gulbransen BD (2018) Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 315:G473–G483
Han X, Tang S, Dong L, Song L, Dong Y, Wang Y, Du Y (2017) Loss of nitrergic and cholinergic neurons in the enteric nervous system of APP/PS1 transgenic mouse model. Neurosci Lett 642:59–65
Reiserer RS, Harrison FE, Syverud DC, McDonald MP (2007) Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav 6:54–65
Sadowski M, Pankiewicz J, Scholtzova H, Ji Y, Quartermain D, Jensen CH, Duff K, Nixon RA, Gruen RJ, Wisniewski T (2004) Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol 63:418–428
Zhang Y, Geng R, Tu Q (2021) Gut microbial involvement in Alzheimer’s disease pathogenesis. Aging (Albany NY) 13:13359–13371
Sun Y, Sommerville NR, Liu JYH, Ngan MP, Poon D, Ponomarev ED, Lu Z, Kung JSC, Rudd JA (2020) Intra-gastrointestinal amyloid-β1-42 oligomers perturb enteric function and induce Alzheimer’s disease pathology. J Physiol 598:4209–4223
Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R et al (2013) Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145:1323–1333
Caputi V, Marsilio I, Filpa V, Cerantola S, Orso G, Bistoletti M, Paccagnella N, De Martin S, Montopoli M, Dall’Acqua S et al (2017) Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol 174:3623–3639
Smith TH, Ngwainmbi J, Grider JR, Dewey WL, Akbarali HI (2013) An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp 78:50688
Hou TT, Yang HY, Wang W, Wu QQ, Tian YR, Jia JP (2018) Sulforaphane inhibits the generation of amyloid-β oligomer and promotes spatial learning and memory in Alzheimer’s disease (PS1V97L) transgenic mice. J Alzheimers Dis 62:1803–1813
Dorey E, Bamji-Mirza M, Najem D, Li Y, Liu H, Callaghan D, Walker D, Lue LF, Stanimirovic D, Zhang W (2017) Apolipoprotein E isoforms differentially regulate Alzheimer’s disease and amyloid-β-induced inflammatory response in vivo and in vitro. J Alzheimers Dis 57:1265–1279
Li M, Liu E, Zhou Q, Li S, Wang X, Liu Y, Wang L, Sun D, Ye J, Gao Y et al (2018) TRPC1 null exacerbates memory deficit and apoptosis induced by amyloid-β. J Alzheimers Dis 63:761–772
Chen S, Jia J (2020) Tenuifolin attenuates amyloid-β42-induced neuroinflammation in microglia through the NF-κB signaling pathway. J Alzheimers Dis 76:195–205
Fa M, Orozco IJ, Francis YI, Saeed F, Gong Y, Arancio O (2010) Preparation of oligomeric beta-amyloid 1–42 and induction of synaptic plasticity impairment on hippocampal slices. J Vis Exp 41:1884
Acknowledgements
The authors acknowledge Dr. Liping Zhu and technician Shaohua Zhang (School of Basic Medicine and Tongji Medical College, Huazhong University of Science and Technology) for excellent technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (grants 31721002, 81920208014, and 31930051 to YL, 32200795 to HL).
Author information
Authors and Affiliations
Contributions
GL, YL, and HL designed the studies and wrote the paper. GL and QY performed the major experiments. HZ, BT, HY, XL, and HL carried out the microscopic imaging, data collection and analysis, and mouse feeding. All authors reviewed and revised the manuscript, especially the authors HZ and HL made significant contributions in the process of paper revision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Animal experimental procedures were conducted following the institutional guidelines and the Animal Care and Use Committee of the animal core facility at Huazhong University of Science and Technology, Wuhan, China.
Consent to participate
Not applicable.
Consent for publication
All authors have read and approved the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Yu, Q., Zhu, H. et al. Amyloid-β mediates intestinal dysfunction and enteric neurons loss in Alzheimer's disease transgenic mouse. Cell. Mol. Life Sci. 80, 351 (2023). https://doi.org/10.1007/s00018-023-04948-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04948-9