Abstract
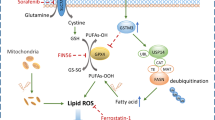
Radiotherapy resistance is a major obstacle to nasopharyngeal carcinoma (NPC) therapy and contributes to tumour recurrence and metastasis. Lipid metabolism is a key regulatory mechanism in cancer biology; however, its role in NPC radiotherapy resistance remains unclear. In this study, we identified hypoxia-inducible lipid droplet-associated protein (HILPDA) as a newly discovered regulator of radioresistance that induces not only lipid droplet (LD) formation but also intracellular lipid remodelling, notably changing mitochondrial cardiolipin (CL) levels. Additionally, we found that the upregulation of CL promotes mitophagy in response to irradiation exposure. Mechanistically, HILPDA inhibits PINK1-mediated CLS1 ubiquitination and degradation. The combination of a mitophagy inhibitor and irradiation significantly increases the radiosensitivity of NPC cells. Human cancer-derived data confirmed that the HILPDA-CLS1 pathway promotes NPC radioresistance. Collectively, these findings suggest that HILPDA plays a critical role in promoting NPC radioresistance and might be targeted to overcome radiotherapeutic resistance in NPC patients in the clinic.








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article.
References
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394(10192):64–80
Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, Han J, Wu G (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104(3):286–293
Mao YP, Tang LL, Chen L, Sun Y, Qi ZY, Zhou GQ, Liu LZ, Li L, Lin AH, Ma J (2016) Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer 35(1):103
Ng WT, Lee MC, Chang AT, Chan OS, Chan LL, Cheung FY, Hung WM, Chan CC, Lee AW (2014) The impact of dosimetric inadequacy on treatment outcome of nasopharyngeal carcinoma with IMRT. Oral Oncol 50(5):506–512
Wu F, Wang R, Lu H, Wei B, Feng G, Li G, Liu M, Yan H, Zhu J, Zhang Y, Hu K (2014) Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol 112(1):106–111
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12(1):31–46
Martinez-Reyes I, Chandel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21(10):669–680
Zhou W, Yao Y, Scott AJ, Wilder-Romans K, Dresser JJ, Werner CK, Sun H, Pratt D, Sajjakulnukit P, Zhao SG, Davis M, Nelson BS, Halbrook CJ, Zhang L, Gatto F, Umemura Y, Walker AK, Kachman M, Sarkaria JN, Xiong J, Morgan MA, Rehemtualla A, Castro MG, Lowenstein P, Chandrasekaran S, Lawrence TS, Lyssiotis CA, Wahl DR (2020) Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat Commun 11(1):3811
Rashmi R, Huang X, Floberg JM, Elhammali AE, McCormick ML, Patti GJ, Spitz DR, Schwarz JK (2018) Radioresistant cervical cancers are sensitive to inhibition of glycolysis and redox metabolism. Cancer Res 78(6):1392–1403
Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, Chen JH, Zhang JT (2011) Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol 2(1):89–98
Tan Z, Xiao L, Tang M, Bai F, Li J, Li L, Shi F, Li N, Li Y, Du Q, Lu J, Weng X, Yi W, Zhang H, Fan J, Zhou J, Gao Q, Onuchic JN, Bode AM, Luo X, Cao Y (2018) Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics 8(9):2329–2347
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, Hamsafar Y, Evans AC, Huang J, Zhou W, Lin X, Ye N, Wanggou S, Chen W, Jing D, Fragoso RC, Dugger BN, Wilson PF, Coleman MA, Xia S, Li X, Sun LQ, Monjazeb AM, Wang A, Murphy WJ, Kung HJ, Lam KS, Chen HW, Li JJ (2022) Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun 13(1):1511
Qu Q, Zeng F, Liu X, Wang QJ, Deng F (2016) Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis 7:e2226
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18(2):153–161
Ertunc ME, Hotamisligil GS (2016) Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res 57(12):2099–2114
Renne MF, Hariri H (2021) Lipid droplet-organelle contact sites as hubs for fatty acid metabolism, trafficking, and metabolic channeling. Front Cell Dev Biol 9:726261
Cruz ALS, Barreto EA, Fazolini NPB, Viola JPB, Bozza PT (2020) Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis 11(2):105
Tirinato L, Marafioti MG, Pagliari F, Jansen J, Aversa I, Hanley R, Nistico C, Garcia-Calderon D, Genard G, Guerreiro JF, Costanzo FS, Seco J (2021) Lipid droplets and ferritin heavy chain: a devilish liaison in human cancer cell radioresistance. Elife 10:e72943
Nistico C, Pagliari F, Chiarella E, Fernandes Guerreiro J, Marafioti MG, Aversa I, Genard G, Hanley R, Garcia-Calderon D, Bond HM, Mesuraca M, Tirinato L, Spadea MF, Seco JC (2021) Lipid droplet biosynthesis impairment through DGAT2 inhibition sensitizes MCF7 breast cancer cells to radiation. Int J Mol Sci 22(18):10102
Povero D, Johnson SM, Liu J (2020) Hypoxia, hypoxia-inducible gene 2 (HIG2)/HILPDA, and intracellular lipolysis in cancer. Cancer Lett 493:71–79
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dancik V, Leshchiner ES, Viswanathan VS, Signoretti S, Choueiri TK, Boehm JS, Wagner BK, Doench JG, Clish CB, Clemons PA, Schreiber SL (2019) A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun 10(1):1617
VandeKopple MJ, Wu J, Auer EN, Giaccia AJ, Denko NC, Papandreou I (2019) HILPDA regulates lipid metabolism, lipid droplet abundance, and response to microenvironmental stress in solid tumors. Mol Cancer Res 17(10):2089–2101
Liu C, Zhou X, Zeng H, Wu D, Liu L (2021) HILPDA is a prognostic biomarker and correlates with macrophage infiltration in pan-cancer. Front Oncol 11:597860
Padmanabha Das KM, Wechselberger L, Liziczai M, De la Rosa Rodriguez M, Grabner GF, Heier C, Viertlmayr R, Radler C, Lichtenegger J, Zimmermann R, Borst JW, Zechner R, Kersten S, Oberer M (2018) Hypoxia-inducible lipid droplet-associated protein inhibits adipose triglyceride lipase. J Lipid Res 59(3):531–541
de la Rosa Rodriguez MA, Deng L, Gemmink A, van Weeghel M, Aoun ML, Warnecke C, Singh R, Borst JW, Kersten S (2021) Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Mol Metab 47:101168
Cui C, Fu K, Yang L, Wu S, Cen Z, Meng X, Huang Q, Xie Z (2019) Hypoxia-inducible gene 2 promotes the immune escape of hepatocellular carcinoma from nature killer cells through the interleukin-10-STAT3 signaling pathway. J Exp Clin Cancer Res 38(1):229
Povero D, Chen Y, Johnson SM, McMahon CE, Pan M, Bao H, Petterson XT, Blake E, Lauer KP, O'Brien DR, Yu Y, Graham RP, Taner T, Han X, Razidlo GL, Liu J (2023) HILPDA promotes NASH-driven HCC development by restraining intracellular fatty acid flux in hypoxia. J Hepatol 79(2):378–393
Schmidt S, Linge A, Zwanenburg A, Leger S, Lohaus F, Krenn C, Appold S, Gudziol V, Nowak A, von Neubeck C, Tinhofer I, Budach V, Sak A, Stuschke M, Balermpas P, Rodel C, Bunea H, Grosu AL, Abdollahi A, Debus J, Ganswindt U, Belka C, Pigorsch S, Combs SE, Monnich D, Zips D, Baretton GB, Buchholz F, Baumann M, Krause M, Lock S, Dktk ROG (2018) Development and validation of a gene signature for patients with head and neck carcinomas treated by postoperative radio(chemo)therapy. Clin Cancer Res 24(6):1364–1374
Roberts MA, Olzmann JA (2020) Protein quality control and lipid droplet metabolism. Annu Rev Cell Dev Biol 36:115–139
Lass A, Zimmermann R, Oberer M, Zechner R (2011) Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50(1):14–27
van Dierendonck X, de la Rosa Rodriguez MA, Georgiadi A, Mattijssen F, Dijk W, van Weeghel M, Singh R, Borst JW, Stienstra R, Kersten S (2020) HILPDA uncouples lipid droplet accumulation in adipose tissue macrophages from inflammation and metabolic dysregulation. Cell Rep 30(6):1811–1822 (e6)
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21(4):204–224
Kondadi AK, Anand R, Reichert AS (2020) Cristae membrane dynamics—a paradigm change. Trends Cell Biol 30(12):923–936
Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15(10):1197–1205
Iriondo MN, Etxaniz A, Varela YR, Ballesteros U, Hervas JH, Montes LR, Goni FM, Alonso A (2022) LC3 subfamily in cardiolipin-mediated mitophagy: a comparison of the LC3A, LC3B and LC3C homologs. Autophagy 18(12):2985–3003
Anton Z, Landajuela A, Hervas JH, Montes LR, Hernandez-Tiedra S, Velasco G, Goni FM, Alonso A (2016) Human Atg8-cardiolipin interactions in mitophagy: specific properties of LC3B, GABARAPL2 and GABARAP. Autophagy 12(12):2386–2403
Chen Q, Zheng W, Zhu L, Liu H, Song Y, Hu S, Bai Y, Pan Y, Zhang J, Guan J, Shao C (2021) LACTB2 renders radioresistance by activating PINK1/Parkin-dependent mitophagy in nasopharyngeal carcinoma. Cancer Lett 518:127–139
Chang HW, Kim MR, Lee HJ, Lee HM, Kim GC, Lee YS, Nam HY, Lee M, Jang HJ, Lee KE, Lee JC, Byun Y, Kim SW, Kim SY (2019) p53/BNIP3-dependent mitophagy limits glycolytic shift in radioresistant cancer. Oncogene 38(19):3729–3742
Yu L, Yang X, Li X, Qin L, Xu W, Cui H, Jia Z, He Q, Wang Z (2021) Pink1/PARK2/mROS-dependent mitophagy initiates the sensitization of cancer cells to radiation. Oxid Med Cell Longev 2021:5595652
Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Patra S, Behera BP, Mishra SR, Bhutia SK (2020) The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin Cancer Biol 66:45–58
Palikaras K, Lionaki E, Tavernarakis N (2018) Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 20(9):1013–1022
Ren M, Phoon CK, Schlame M (2014) Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res 55:1–16
Zhang J, Guan Z, Murphy AN, Wiley SE, Perkins GA, Worby CA, Engel JL, Heacock P, Nguyen OK, Wang JH, Raetz CR, Dowhan W, Dixon JE (2011) Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab 13(6):690–700
Chen BB, Coon TA, Glasser JR, Zou C, Ellis B, Das T, McKelvey AC, Rajbhandari S, Lear T, Kamga C, Shiva S, Li C, Pilewski JM, Callio J, Chu CT, Ray A, Ray P, Tyurina YY, Kagan VE, Mallampalli RK (2014) E3 ligase subunit Fbxo15 and PINK1 kinase regulate cardiolipin synthase 1 stability and mitochondrial function in pneumonia. Cell Rep 7(2):476–487
Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH (2012) The battle against nasopharyngeal cancer. Radiother Oncol 104(3):272–278
Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA (2022) The role of lipids in cancer progression and metastasis. Cell Metab 34(11):1675–1699
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H (2018) JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27(1):136–150 (e5)
Duncan AL, Robinson AJ, Walker JE (2016) Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc Natl Acad Sci USA 113(31):8687–8692
Tsuchiya H, Shinonaga R, Sakaguchi H, Kitagawa Y, Yoshida K, Shiota G (2022) NEAT1 confers radioresistance to hepatocellular carcinoma cells by inducing PINK1/parkin-mediated mitophagy. Int J Mol Sci 23(22):14397
Fang Y, Zhan Y, Xie Y, Du S, Chen Y, Zeng Z, Zhang Y, Chen K, Wang Y, Liang L, Ding Y, Wu D (2022) Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology 75(6):1386–1401
Belikova NA, Jiang J, Tyurina YY, Zhao Q, Epperly MW, Greenberger J, Kagan VE (2007) Cardiolipin-specific peroxidase reactions of cytochrome C in mitochondria during irradiation-induced apoptosis. Int J Radiat Oncol Biol Phys 69(1):176–186
Tu C, Xiong H, Hu Y, Wang W, Mei G, Wang H, Li Y, Zhou Z, Meng F, Zhang P, Mei Z (2020) Cardiolipin synthase 1 ameliorates NASH through activating transcription factor 3 transcriptional inactivation. Hepatology 72(6):1949–1967
Acknowledgements
We gratefully acknowledge Dr. Wei Guo from the College of Pharmacy, Third Military Medical University (Army Medical University) for her kind help in providing EGFP-LC3 and RFP-Mito plasmids.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China, including 81874072 (H.L.) and 81772647 (H.L.); Chongqing Science and Technology Commission 4139Z2395(H.L.).
Author information
Authors and Affiliations
Contributions
JO, GX and HL designed the study and wrote the paper. YZ, CP and CZ performed the experiments. YW, PW, YC, JW, YH and CL analysed the data. JO, GX, YZ and CP revised the manuscript. All authors contributed to this manuscript. All authors had full access to the data and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the 1964 Declaration of Helsinki. The protocol for IHC staining of patient tissues was approved by the Ethics Committee of the First Affiliated Hospital (Southwest Hospital), the Third Military Medical University (Army Medical University), and all patients or family members involved provided written informed consent.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Pang, C., Zhang, C. et al. HILPDA-mediated lipidomic remodelling promotes radiotherapy resistance in nasopharyngeal carcinoma by accelerating mitophagy. Cell. Mol. Life Sci. 80, 242 (2023). https://doi.org/10.1007/s00018-023-04891-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04891-9